Abstract
Motivational approaches to depression emphasize the role of dysfunctional motivational dynamics, particularly diminished reward and incentive processes associated with anhedonia. A study examined how anhedonic depressive symptoms, measured continuously across a wide range of severity, influenced the physiological mobilization of effort during a cognitive task. Using motivational intensity theory as a guide, we expected that the diminished incentive value associated with anhedonic depressive symptoms would reduce effort during a “do your best” challenge (also known as an unfixed or self-paced challenge), in which effort is a function of the value of achieving the task’s goal. Using impedance cardiography, two cardiac autonomic responses were assessed: pre-ejection period (PEP), a measure of sympathetic activity and our primary measure of interest, and respiratory sinus arrhythmia (RSA), a measure of parasympathetic activity. As expected, PEP slowed from baseline to task as anhedonic depressive symptoms increased (as measured with the DASS Depression scale), indicating diminished effort-related sympathetic activity. No significant effects appeared for RSA. The findings support motivational intensity theory as a translational model of effort processes in depression and clarify some inconsistent effects of depressive symptoms on effort-related physiology found in past work.
Keywords: effort, depression, anhedonia, motivational intensity, autonomic nervous system, impedance cardiography
Anhedonia—diminished interest or pleasure in daily activities—is one of the defining features of depression, both major depressive disorder and the subclinical continuum of dysphoric and depressive symptoms. Although anhedonia has traditionally been viewed as a deficit in emotional responding, recent research has emphasized how aspects of motivation, such as the processing and weighting of rewards and incentives, underlie depressive anhedonia (e.g., Pizzagalli et al., 2008; Treadway & Zald, 2011).
In the present research, we apply motivational intensity theory (Brehm & Self, 1989), a general model of effort, to understand how anhedonic depressive symptoms affect cardiac processes during motivational challenges. Broadly speaking, the theory suggests two pathways by which the onset of depression could affect effort: changes in appraised task difficulty (goals seem easier or harder to achieve) and changes in appraised goal importance (the incentives at stake seem more or less valuable). Prior applications of this theory (for reviews, see Brinkmann & Franzen, in press; Gendolla, Brinkmann, & Silvestrini, 2012) have obtained solid evidence for the first pathway (changes in task difficulty), but evidence for the second is complex and inconsistent. We thus examined whether anhedonic depressive symptoms can affect effort, measured with cardiac autonomic changes during an active coping challenge, by affecting the perceived importance of achieving a goal. By clarifying the inconsistent findings to date on whether this is one route by which depressive symptoms affect effort, we can thus expand the emerging analysis of effort and depression in terms of motivational intensity theory (Brinkmann & Franzen, in press).
Depression and Cardiovascular Markers of Motivation
To date, much of the research on depression and cardiovascular markers of motivation has assumed simple main effects. Drawing from the tradition of work on cardiovascular reactivity and health (Phillips & Hughes, 2011), several researchers have proposed global main effects of depression. In some cases, researchers have proposed that depression is associated with blunted cardiac reactivity in response to challenges and stressors (e.g., Phillips, 2011; Salomon et al., 2009; Schwerdtfeger & Rosenkaimer, 2011). In other cases, researchers have proposed that depression is associated with exaggerated reactivity (Light et al., 1998). The typical method in this literature uses standard tasks—such as mental arithmetic, cold pressor, or public-speaking stress tasks—that are fixed and invariant, in that they don’t manipulate variables (e.g., incentives, task difficulty, expected rewards) known to affect motivation. As a result, it isn’t surprising that researchers have found inconsistent, task-specific effects of depression on biological markers of motivational engagement (Rottenberg, 2007). These invariant tasks surely vary in key motivational parameters (e.g., mental arithmetic may be easier than a cold pressor task, and public speaking may be more important than mental arithmetic), but their effects are obscured when each task holds the parameters constant at an unknown value.
A more productive approach is to apply basic-science models of motivation to the problem of depression (Treadway & Zald, 2011). By translating an established model of effort, the field can use established concepts and mature paradigms to illuminate the potentially intricate effects of depression on cardiovascular markers of effort. In their line of work, Brinkmann, Gendolla, and their colleagues (Brinkmann & Franzen, in press; Gendolla et al., 2012) have applied motivational intensity theory to the problem of depressive symptoms. Motivational intensity theory suggests that the mobilization of bodily resources to confront a challenge is a joint function of two factors: the importance of the goal at stake and the difficulty of the actions necessary to attain it (Brehm & Self, 1989; Wright, 1996). Goal importance, the first factor, sets a ceiling on the amount of effort people are willing to expend—this limit is often called the level of potential motivation (Wright, 2008) because it reflects how much effort people could mobilize if necessary. Task difficulty, the second factor, determines the actual amount of effort people mobilize. When task difficulty is low, effort will be low: even if a goal is important (and potential motivation is thus quite high), ramping up the body to accomplish something easy is unnecessary and wasteful. As task difficulty increases, effort increases as well. Eventually, however, effort will decline. People might hit the ceiling of potential motivation (they could achieve the goal with even more effort but the goal isn’t valuable enough to merit the effort) or the task might appear impossible (no amount of effort will result in attaining the goal). In both cases, it is better to withdraw effort and thus conserve one’s resources for more fruitful goals and sources of reward (Richter, 2013).
Evidence for Task Difficulty Effects
Motivational intensity theory offers some new insights into how depression could affect effort. First, depression could affect perceptions of a task’s difficulty. Negative affect (Gendolla, 2000; Gendolla, Abele, & Krüsken, 2001) and low perceived ability (Wright, 1998; Wright & Dill, 1993) both make tasks seem harder, so it seems likely that depressive symptoms can affect effort by increasing people’s perceptions of a task’s difficulty. Several studies have consistently supported this prediction. In two experiments, dysphoric and nondysphoric participants worked on cognitive tasks that were either fairly easy or challenging but feasible (Brinkmann & Gendolla, 2008, Study 1 and 2). Effortful engagement was measured with increased systolic blood pressure (SBP) reactivity from baseline to task. All else equal, increased activity of the sympathetic branch of the autonomic nervous system increases SBP (Mohrman & Heller, 2010), and SBP reactivity has been widely used in effort research (Wright, 1996). Nondysphoric participants showed low SBP reactivity when the task was easy but high SBP reactivity when the task was hard—as one would expect, they put forth more effort as the task got harder. Dysphoric participants, however, showed high SBP reactivity when the task was easy, which they found subjectively harder as a result of their negative mood, but showed lower SBP reactivity when the task was hard. The easy task thus struck them as relatively harder, and the hard task struck them as less feasible. Together, the experiments support the predictions of motivational intensity theory, and they illustrate the importance of a dynamic analysis of depression and effort instead of an assumption of simple main effects.
Evidence for Goal Value Effects
Second, depression could affect effort by affecting perceptions of a goal’s value. The motivational analysis of anhedonia contends that anhedonia involves diminished incentive value: goals are less rewarding and appealing (Pizzagalli et al., 2008; Treadway & Zald, 2011). It thus seems likely that depression can affect effort by making goals seem less desirable, thereby lowering the ceiling of potential motivation (Wright, 2008).
Tasks with uncertain difficulty levels
Evidence for this route is considerably more complex. Motivational intensity research has two common paradigms to evaluate if a factor affects potential motivation. One approach uses uncertain task difficulty: when a task has a fixed level of difficulty but people don’t know what it will be, they will mobilize effort up to the level of potential motivation (Richter & Gendolla, 2006, 2007, 2009b). This is a rational use of effort because people are “preparing for the worst”: by mobilizing as much effort as the task merits in light of its reward value, people are ready for all levels of difficulty equal to or lower than what the goal deserves. Research can thus use uncertain-difficulty tasks to “diagnose” whether a factor affects potential motivation. If a factor (e.g., money) increases effort when difficulty is uncertain, then it is affecting potential motivation.
Consistent with a motivational intensity analysis, several studies have found that depressive symptoms reduce effort for uncertain-difficulty tasks. In one experiment (Brinkmann, Schüpbach, Joye, & Gendolla, 2009, Study 2), people learned they could earn 10 Swiss Francs if they successfully met the standard for a memory task of uncertain difficulty. Measures of sympathetic activation—higher systolic blood pressure (SBP) and faster cardiac pre-ejection period (PEP), a more precise measure of sympathetic activity (Kelsey, 2012)—revealed that dysphoric participants showed less effort engagement from baseline to task compared to nondysphoric participants. In another experiment (Brinkmann & Franzen, 2013), participants expected to receive either 0, 5, or 15 Swiss Francs if they successfully met the standard for a task with an uncertain difficulty level and unknown performance standard. As expected, nondysphoric participants showed faster PEP values—reflecting stronger sympathetic activation—as the reward increased, but dysphoric participants were generally unaffected by the anticipated reward, suggesting diminished incentive value. All told, experiments using the uncertain-difficulty paradigm support the view that depressive symptoms can reduce effort by reducing the value of rewards.
Tasks with unfixed difficulty levels
The second paradigm for evaluating if a factor affects potential motivation contradicts the first. Research commonly uses unfixed tasks—also known as self-paced, piece-rate, and do-your-best tasks—to evaluate whether a factor influences goal value (Wright, Killebrew, & Pimpalapure, 2002). Unfixed tasks lack an aspect of difficulty: people can do as much or as little as they like, depending on how much the outcome matters to them. Some unfixed tasks have a piece-rate reward structure, such as when people receive a few cents for each correct response (e.g., Richter, 2010). Other unfixed tasks have a general achievement standard: people are told simply to do their best and accomplish as much as they can, and effort reflects the value of doing well (e.g., Silvia, 2012, Study 1). Because effort for unfixed tasks solely reflects the goal’s value (Wright et al., 2002), these tasks are widely used to diagnose whether a factor affects effort by affecting a goal’s value (Wright, 2008).
One would expect, given the line of work with uncertain-difficulty tasks and the reasoning behind unfixed tasks, that depressive symptoms would reduce effort for unfixed tasks. But to the contrary, several studies have found that people with elevated depressive symptoms try harder for unfixed tasks. In one experiment, dysphoric participants showed higher SBP reactivity during the early period of an unfixed task (Brinkmann & Gendolla, 2007, Study 1). In another, dysphoric participants showed higher SBP reactivity for the duration of an unfixed task (Brinkmann & Gendolla, 2007, Study 2). In a third, dysphoric participants showed higher SBP reactivity in a control condition, but not in a condition that provided a mood-discounting cue (Brinkmann, Grept, & Gendolla, 2012). The unfixed-task experiments thus show that dysphoria promotes stronger effort, but the uncertain-difficulty experiments show that dysphoria promotes weaker effort.
Why do these literatures conflict? A possible reconciliation comes from research by Richter (2010), who demonstrated that subtle contextual features can convert unfixed tasks into fixed-difficulty tasks. In his research (Richter, 2010, Study 2), people worked on an unfixed task that provided a small or a moderate monetary reward for each correct response. In one condition, people were asked before the task to rate their perceptions of the task’s level of demand and difficulty; in another, people were asked to rate before the task their perceptions of the reward’s value. This simple manipulation of pre-task context changed the nature of the unfixed task. When people were asked about reward value, the task affected effort like the typical unfixed task—effort increased as the reward value increased. But when people were asked about difficulty, effort was insensitive to reward amount. In short, an unfixed task can “behave” like a fixed-difficulty task if people are oriented to information that frames the task in terms of difficulty, demands, and challenges.
The Present Research
In the present research, we sought evidence for an effect of depressive symptoms on potential motivation in unfixed tasks. Consistent with an anhedonic effect of depression, we expected that effort-related cardiac activity would become weaker as depressive symptoms increased. Our primary measure of interest was PEP. As noted earlier, motivational intensity theory has emphasized measures of beta-adrenergic sympathetic impact on the heart, such as systolic blood pressure and PEP. Sympathetic activity has an inotropic influence on the heart, increasing the force of ventricular contraction (Drew & Sinoway, 2012; Mohrman & Heller, 2010). The increase in contractility is reflected in the decreased time between the onset of contraction (indicated by the start of electrical depolarization of the heart) and the ejection of blood (indicated by the opening of the aortic valve). A large psychophysiological literature supports the use of PEP as a measure of beta-adrenergic sympathetic influence on the heart in general (e.g., Cacioppo et al., 1994; Kelsey, 2012; Obrist, Light, James, & Strogatz, 1987; Schächinger et al., 2001) and in the study of effort in particular (see Wright & Gendolla, 2012). PEP generally covaries with SBP, the more common measure, but it is much more precise and is thus preferred over SBP in much modern research (Richter, Friedrich, & Gendolla, 2008; Richter & Gendolla, 2009b).
Our study measured the activity of both the sympathetic and parasympathetic branches of the autonomic nervous system. Motivational intensity theory has almost exclusively emphasized sympathetic processes, but parasympathetic processes are intriguing for the study of effort and depression. Parasympathetic activity—primarily quantified as respiratory sinus arrhythmia (RSA)—has attracted a great deal of attention among depression researchers in a range of contexts, such as experimental research on depression (e.g., Salomon et al., 2009; Yaroslavsky, Rottenberg, & Kovacs, 2013), studies aimed at predicting response to treatment (e.g., Rottenberg, Salomon, Gross, & Gotlib, 2005), and health research on depression’s role in cardiovascular disease (e.g., Kemp et al., 2010). We did not have predictions concerning RSA, but it seemed potentially fruitful in light of the large literature on RSA’s links to depression (Rottenberg, 2007) and to self-regulatory processes (Segerstrom & Nes, 2007).
Method
Participants
The final sample consisted of 131 adults—85 women (65%), 46 men (35%)—who volunteered to participate either as part of a research option in a psychology class or for $10. The sample was primarily young (mean age = 19.37 years, SD = 1.84, range from 18 to 30). Based on self-reported race and ethnicity, the sample was diverse: 59% European American, 28% African American, 12% Hispanic or Latino, and 6% Asian or Pacific Islander (people could pick more than one category or decline to pick any). The average body mass index (BMI), based on self-reported height and weight, was 24.42 (SD = 4.72), placing the sample at the cusp between normal weight and overweight. Six cases had been collected but excluded because of cardiac problems mentioned after the session (e.g., congenital defects or surgeries), an inability to get strong signals, or hardware and software malfunctions.
Procedure
The project was approved by our institution’s IRB. After providing informed consent, the participants learned that the study was about how the body responded physically during mental challenges, particularly how different aspects of personality might relate to physiological markers of effort and engagement. They expected to complete a wide range of self-report questionnaires and to work on a challenging cognitive task. After the experimenter placed the electrodes, the participants sat quietly and completed a long series of innocuous self-report scales that measured demographic information and personality variables. The scales and tasks were presented using MediaLab and DirectRT (Empirisoft, NY). The scales took roughly 10 minutes to complete. The middle 5 minutes of this period were used for baseline readings. Unlike a “relaxation baseline,” this baseline period shares irrelevant cognitive, postural, and motor factors with the task period (e.g., sitting upright, viewing a monitor, reading, and pressing keyboard keys) and thus eliminates them as confounds.
The parity task
For the mental challenge, we used the parity task (Wolford & Morrison, 1980), a cognitive task that involves deciding whether two numbers have the same parity (i.e., both are odd or both are even) or a different parity (i.e., one is odd and the other is even). In this task, people see a word in the center of the screen that is flanked by two digits, such as “2 CHAIR 8.” People are told to ignore the word and make the parity judgment. This task is challenging because ignoring centrally-presented words is hard and making parity judgments is unusual (Aquino & Arnell, 2007; Harris & Pashler, 2004). This task has been effective in our past research (Silvia, Eddington, Beaty, Nusbaum, & Kwapil, 2013; Silvia & Phillips, 2013).
We used four numbers (2, 3, 5, 8) and 16 neutral nouns (e.g., BOAT, CHAIR, MARKET). Each trial started with a fixation cross (350 ms) followed by a parity item. The black text (28 pt Tahoma) was displayed on a white background. The parity item remained on screen until people responded. After a 750 ms intertrial interval, the next trial began. The task terminated after 5 minutes. Responses were collected with a DirectIN high-speed keyboard (Empirisoft, NY), which has a timing accuracy of under 1 ms. A green key was mapped to “same parity,” and a red key was mapped to “different parity.”
As in our past work (Silvia, Eddington et al., 2013), the parity task was “unfixed” in difficulty (Wright et al., 2002). Each trial remained on the screen until people responded and then terminated immediately, so people could work at their own pace. Responding more quickly thus allows people to potentially get more correct. We took care to avoid turning the task into a “quasi-fixed task,” which can happen if a fixed standard for performance is implied (e.g., try to get at least 100 right) or if people are asked questions about task difficulty beforehand (Richter, 2010). To avoid this problem, we didn’t ask questions about perceptions of difficulty or other task features before the task, and we emphasized that the task’s goal was for people to do their best and to get as many correct as they could. Likewise, to avoid creating an “uncertain difficulty” task, we instructed participants about the task’s length, features, sample items, and goal beforehand. People knew that the task lasted for exactly 5 minutes, that they could work at their own pace, and that they should try to get as many correct as possible.
The parity task yielded two related performance outcomes: the number of correct responses and response times (in ms) for correct responses. After the task, people completed two self-report items related to the task and their performance. They were asked “In your opinion, how easy or hard was this task?” (1 = very easy, 7 = very hard) and “In your opinion, how well do you think you did on this task?” (1 = very poorly, 7 = very well).
Assessment of depression
After the parity task, we assessed depressive symptoms with the Depression subscale of the 21-item form of the Depression Anxiety Stress Scales (DASS; Lovibond & Lovibond, 1995). The DASS Depression scale was our primary measure because it emphasizes anhedonic symptoms—items include “I was unable to become enthusiastic about anything,” “I felt that I had nothing to look forward to,” and “I found it difficult to work up the initiative to do things”—and works well with both clinical and non-clinical samples (Antony, Beiling, Cox, Enns, & Swinson, 1998; Brown, Chorpita, Korotitsch, & Barlow, 1997; Lovibond, 1998). The DASS Depression scale has 7 items that refer to people’s experiences during the past week. People respond to each using a 4-point scale (0 = did not apply to me at all, 4 = applied to me very much, or most of the time), and the items were averaged for an overall score.
The sample as a whole showed few symptoms (M = .59, SD = .63), but the range was wide (from 0 to 2.86). In Antony et al.’s (1998) study of the DASS in clinical samples, adults diagnosed with major depressive disorder had DASS Depression scores of 2.14, and adults diagnosed with social phobia, panic disorder, and obsessive-compulsive disorder had scores of around 1. About 20% of our sample had scores greater than 1, and 6% had scores greater than 2.1
Physiological assessment
We measured cardiac activity with a Mindware Bionex hardware system (Mindware, Gahanna, OH). An electrocardiogram (ECG) was obtained with a modified Lead-II configuration: the electrodes were placed on the left and right lowest ribs and the right clavicle. An impedance cardiogram (ICG) was obtained with disposable spot electrodes in a standard tetrapolar configuration: the two receiving electrodes were placed on the front of the body (an upper one 4 cm above the suprasternal notch, and a lower one at the base of the sternum on the xiphoid process), and the two sending electrodes were placed on the back (one 4 cm above the horizontal plane of the first and one 4 cm below the horizontal plane of the xiphoid). The signals were sampled at 1000 Hz and processed offline using bandpass filters (ECG and dZ/dt, .5 to 45 Hz; Z0, 10 to 45 Hz).
The baseline period and task period were each five minutes. We divided the 10 minutes of interest into 60-second epochs, and the physiological values were calculated for each epoch. For PEP, ensemble averages of the ECG and ICG dZ/dt waveforms (Kelsey et al., 1998) were used. PEP was computed as the difference between the ECG Q-point (denoting the onset of ventricular depolarization; Berntson, Lozano, Chen, & Cacioppo, 2004) and the dZ/dt B-point (denoting the opening of the aortic valve and hence left ventricular ejection onset; Lozano et al., 2007), which were automatically identified by the software (Mindware, Gahanna, OH). When corrected manually in a small number of cases, B-points were identified as the notch or inflection prior the dz/dt wave’s final upstroke (see Sherwood et al., 1990). For RSA, spectral methods were used to compute high-frequency heart rate variability (HRV) in the respiratory frequency range (0.12–0.40 Hz). Respiration rate (in cycles per minute) was calculated from the ICG Z0 thoracic impedance signal (see Ernst, Litvack, Lozano, Cacioppo, & Berntson, 1999). Spectral analysis can identify the variation in Z0 caused by respiration (see Ernst et al., 1999, for details), and it yields scores that are highly similar to spirometric respiration measures (de Geus, Willemsen, Klaver, & van Doornen, 1995; Ernst et al., 2009; Houtveen, Groot, & de Geus, 2006).
Results
Analytic Approach and Descriptive Statistics
As in our recent work (Silvia, Eddington et al., 2013), we analyzed the data using multilevel models, which simultaneously estimate within-person effects (e.g., whether PEP changes from baseline to task), between-person effects (e.g., whether depressive symptoms have a main effect on PEP), and their interactions (e.g., whether depressive symptoms moderate the change in PEP from baseline to task). Multilevel models have some additional virtues, such as the ability to have within-person covariates (e.g., controlling for respiration at the within-person, epoch-by-epoch level), to control for initial value effects, and to flexibly accommodate occasional missing values (see Kristjansson, Kircher, & Webb, 2007; Llabre, Spitzer, Siegel, Saab, & Schneiderman, 2004).
In our models, depressive symptoms (DASS scores) were the between-person (Level 2) predictor. DASS scores were centered at the sample’s overall mean. Time period (baseline vs task) was the within person (Level 1) predictor. Time was scored as 0 (for the 5 60-sec baseline epochs) and 1 (for the 5 60-sec task epochs), so each person had 10 time scores. Time was left uncentered (see Singer & Willett, 2003), so the intercept for time represents values during the baseline period. The models were estimated using Mplus 7.11. The intercepts and slopes were estimated as random effects, and the random intercepts and slopes were allowed to covary to capture possible initial value effects. RSA varies with respiration rate (Berntson, Cacioppo, & Quigley, 1993; Grossman & Taylor, 2007), so the RSA models included respiration rate as a within-person (Level 1) predictor. The reported regression weights are unstandardized.
Depressive Symptoms and PEP
Did depressive symptoms influence PEP, our primary physiological marker of effort, during the parity task? A multilevel model found a significant within-person main effect of time, b = −.59, SE = .24, p = .013: for the sample as a whole, PEP was faster during the task than the baseline period, reflecting greater sympathetic activation. There was also a between-person main effect of DASS scores on the PEP intercept, b = 3.65, SE = 1.55, p = .019: people with higher depressive symptoms had slower PEP baseline values.
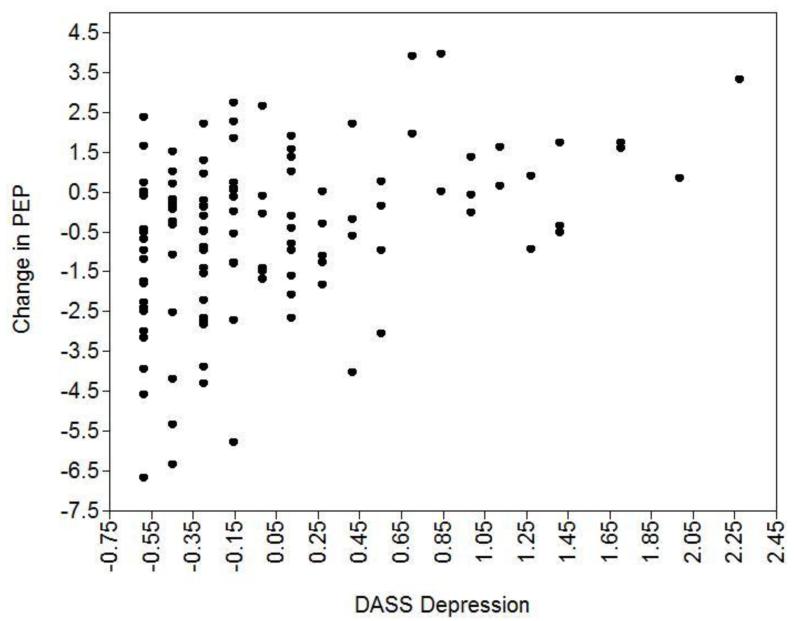
Finally, and most relevant to our hypotheses, there was a significant interaction, b = 1.24, SE = .31, p < .001, indicating that depressive symptoms moderated the change in PEP from baseline to task. The pattern supports our predicted effects: people with low DASS scores had faster PEP scores during the task, reflecting greater effortful engagement, whereas people with high DASS scores had slower PEP scores during the task. Figure 1 depicts this interaction as a scatterplot: as DASS scores increased, PEP change scores became more positive, reflecting less sympathetic activation among people with high depressive symptoms.2
Figure 1.
The relationship between DASS Depression scores and change in PEP from baseline to task.
Depressive Symptoms and RSA
We estimated a similar model for RSA, our secondary outcome. For this model, respiration rate was included as a within-person covariate that was centered at each person’s own mean. At the within-person level, there was no main effect of time, b = .04, SE = .05, p = .463, so RSA did not change for the sample as a whole from baseline to task. Respiration rate, not surprisingly, predicted RSA, b = −.06, SE = .01, p < .001: RSA declined as respiration rate increased, which is a common effect in the literature (Berntson et al., 1993). At the between-person level, depressive symptoms did not predict either the RSA intercept (b = .02, SE = .20, p = .915) or change in RSA from baseline to task (b = .01, SE = .09, p = .876). In short, consistent with motivational intensity theory’s emphasis on sympathetic processes and effort, we observed the predicted pattern of effects for PEP but not for RSA.3
Task Performance
The parity task yielded two performance scores: the number of correct responses and response times for correct responses. Table 2 shows the descriptive statistics for performance and for people’s self-ratings of the task’s difficulty and their own performance. Not surprisingly, people who got more items correct—the parity task’s goal—viewed the task as less hard (r = −.35, p < .001) and their performance as relatively better (r = .43, p < .001).
Table 2. Task Performance and Self-Reported Task Perceptions.
| Mean | SE | 1. | 2. | 3. | 4. | |
|---|---|---|---|---|---|---|
| Number of Correct Items | 132.55 | 1.74 | 1 | |||
| Response Time | 1054 | 28 | −.90 | 1 | ||
| Self-reported task difficulty | 2.86 | .12 | −.35 | .20 | 1 | |
| Self-reported performance quality | 4.83 | .11 | .43 | −.27 | −.54 | 1 |
Note. n = 131. Response times are rounded to the nearest millisecond. People responded to the self-report items after the task using 7-point scales. Higher values indicate higher perceived task difficulty and better perceived performance. All of the correlations are significant, p < .03.
How did depression symptoms influence performance? For objective performance, DASS scores did not significantly predict the number of correct items (r = −.05, p = .580) or response times (r = .05, p = .602). For self-reports, DASS scores didn’t predict how hard people rated the task (r = .05, p = .549), but they did predict people’s perceptions of their own performance (r = .20, p = .024). As depressive symptoms increased, people rated their task performance more negatively.
Finally, did task performance covary with physiological activity during the task? We estimated models in which the performance scores and self-report items predicted change in physiological outcomes from baseline to task. For PEP, there were no significant effects for the number of correct items (b = −.01, SE = .01, p = .282), response times (b = .001, SE = .001, p = .247), self-reported difficulty (b = .02, SE = .19, p = .913), or self-reported performance (b = −.17, SE = .19, p = .357). For RSA, as with PEP, there were no significant effects for the number of correct items (b = −.01, SE = .01, p = .385), response times (b = .001, SE = .001, p = .232), or self-reported difficulty (b = −.01, SE = .03, p = .627). One marginal effect, for self-reported performance, appeared, (b = −.06, SE = .03, p = .054): people who felt they did better had relatively larger declines in RSA from baseline to task. All told, sympathetic and parasympathetic changes were generally unrelated to objective and subjective measures of performance.4
Discussion
Motivational intensity theory (Brehm & Self, 1989), a versatile and increasingly prominent model of effort (Wright & Gendolla, 2012), has been extended to a wide range of problems. Motivational deficits in depression have received extensive attention in recent applications of the theory (Brinkmann & Franzen, in press; Gendolla et al., 2012), but the findings are not altogether consistent. To expand this emerging perspective and to shed some light on the inconsistent findings, the present research examined the effects of anhedonic depressive symptoms on cardiac autonomic markers of effort during an unfixed task.
According to the theory and past research, effort during unfixed, do-your-best tasks is a function of the importance of success (Wright, 2008; Wright et al., 2002). One would thus expect that people high in anhedonia would expend less effort because of the diminished incentive value of the goals and rewards at stake. The present study supported our predictions. Changes in PEP, an index of beta-adrenergic sympathetic influence on the heart (Kelsey, 2012) and a popular measure of effort-related cardiac activity, varied as a function of depressive symptoms. For people with low DASS scores, PEP became faster, reflecting increased effort; for people with high DASS scores, however, PEP became slower, reflecting sympathetic withdrawal.
Few parasympathetic effects were found. For RSA, a frequency-domain metric, there were no main effects or interactions. For RMSSD, a time-domain metric, there was only a marginally significant main effect of time: RMSSD was higher in the task period than the baseline. Several recent studies of motivational intensity theory have included parasympathetic measures, largely for exploratory purposes (e.g., Richter, 2010; Silvia, Eddington et al., 2013). Motivational intensity theory has focused almost exclusively on sympathetic processes, and the large literature to date offers strong support for those predictions. It isn’t clear how the theory would be expanded to address parasympathetic processes, but in some respects findings such as the present results—strong and expected sympathetic effects and no parasympathetic effects—indicate that the heavy focus on sympathetic processes is warranted. Other literatures, however, have proposed that HRV is a core marker of self-regulation, effort, and self-control (e.g., Graziano & Derefinko, 2013; Segerstrom et al., 2012). Ironically, these literatures are a mirror image of the motivational intensity literature: they focus heavily on HRV yet ignore the large literature on sympathetic markers of effort. Finding points of agreement or conceptual integration is an important task for future work.
Essentially no effects were found for task performance. Neither depressive symptoms nor physiological outcomes significantly predicted objective task performance. People with higher DASS scores thought they did worse on the parity task, but objective performance was unaffected. Many tests of motivational intensity theory find correlations between physiological markers of effort and behavioral measures of performance (e.g., Silvia, McCord, & Gendolla, 2010); many more studies, including most of our group’s studies, find only weak or null correlations with performance (e.g., Silvia, Jones, Kelly, & Zibaie, 2011a, 2011b; Silvia, Kelly, Zibaie, Nardello, & Moore, 2013; Silvia, Moore, & Nardello, in press). How hard people try and how well they actually do are quite different. High effort doesn’t always foster high performance, and high performance doesn’t always require the mobilization of high effort. Indeed, high effort often coincides with poor performance, as in cases of “compensatory effort” (Hockey, 1997) brought about by fatigue, sleep deprivation, or low ability (Wright, 1998; Wright & Stewart, 2012).
Reconciling Past Research
The present study provides important support for the application of motivational intensity theory to depression. If depressive symptoms reduce the incentive value of rewards, then they should reduce effort for uncertain-difficulty tasks and for unfixed-difficulty tasks. The evidence for uncertain-difficulty tasks, reviewed earlier, strongly supports the theory’s predictions, but the evidence for unfixed-difficulty tasks does not. We found, as expected, that people higher in depressive symptoms showed weaker sympathetic control of the heart, but other experiments have found the opposite (Brinkmann et al., 2012; Brinkmann & Gendolla, 2007).
What is behind this discrepancy? Richter’s (2010) research on task context offers the most likely explanation for the curious findings in past research. As described earlier, his research found that unfixed tasks could behave like fixed-difficulty tasks if the task context framed the task in terms of difficulty, challenge, and ability. In one past study (Brinkmann et al., 2012), for example, the participants rated the task on measures of difficulty and demand before working on it, something that Richter (2010, Study 2) found made an unfixed task behave like a fixed one. Similarly, several studies administered measures of positive and negative affect before task performance (Brinkmann et al., 2012; Brinkmann & Gendolla, 2007, Study 1). Although mood effects are clearly complex (Gendolla, 2000), calling attention to them may have increased their use in subjective judgments of difficulty. If so, then the heightened negative mood in the dysphoric conditions would increase effort by making the task seem harder. Not every past experiment fits this task context reinterpretation—one experiment measured neither moods nor task difficulty (Brinkmann & Gendolla, 2007, Study 2)—but the present findings, coupled with the theory’s reasoning for unfixed tasks, suggests that future work would be much more likely to find diminished effort, not increased effort, due to depressive symptoms for such tasks.
Methodological Issues
It’s worth noting a few methodological issues. Concerning strengths, the present study extended past research in some useful ways, such as by using a fairly large sample (n = 131) compared to past work and by measuring depressive symptoms along a wide, continuous distribution, not as discrete high-versus-low groups. Second, our measure of depression, the DASS, expands this literature beyond the exclusive use of the CES-D (Brinkmann & Franzen, in press). And third, we assessed both branches of the autonomic nervous system. Concerning weakness, measures of contractility, such as PEP, are affected not only by changes in autonomic influences but also by ventricular preload and afterload (Obrist et al., 1987). We can rule out preload as a possible confound because interbeat intervals were steady from baseline to task (a non-significant change of only 5 ms; see Table 1), so diastolic filling time didn’t increase. Afterload, however, cannot be definitively ruled out due to the lack of measures of blood pressure, particularly diastolic blood pressure. Nevertheless, evidence for afterload-biased PEP changes with stationary participants during mental tasks is rare, as such effects are more likely when the task evokes strong alpha-adrenergic sympathetic changes in the peripheral vasculature (e.g., cold pressor tasks; Obrist et al., 1987).
Table 1. Descriptive Statistics.
| Variable | Mean | SE |
|---|---|---|
| DASS Depression Scale | .59 | .06 |
| PEP (Baseline) | 120.52 | .92 |
| PEP (Task) | 120.29 | .99 |
| RSA (Baseline) | 6.22 | .12 |
| RSA (Task) | 6.22 | .11 |
| IBI (Baseline) | 773.92 | 11.12 |
| IBI (Task) | 778.82 | 10.57 |
| Respiration Rate (Baseline) | 17.95 | .24 |
| Respiration Rate (Task) | 18.41 | .26 |
Note. n = 131. PEP = pre-ejection period (in ms); RSA = respiratory sinus arrhythmia (in ms2); IBI = interbeat interval (in ms); respiration rate is in cycles per minute. These values are raw descriptive statistics. Due to estimation methods and occasional missing data, the values will vary from the maximum-likelihood-based values of intercepts and slopes estimated in the multilevel models.
Summary
Taken together, the present study and past research illustrate the value of motivational intensity theory for understanding depressive effort deficits. Much past research has asserted simple main effects of depression on physiological outcomes, but the inconsistent main effects clearly indicate that a more complex and comprehensive model of effort, such as motivational intensity theory (Brehm & Self, 1989), is needed to understand how motivational processes go awry in depression. Depressive symptoms influence effort via both of the theory’s pathways—they make tasks seem harder (Brinkmann & Gendolla, 2008), and they make goals and rewards seem less appealing. As a result, the theory can offer new insights and predictions about how depression impairs the mobilization of effort in the body.
Acknowledgments
We thank Christina Chai Chang, Emily Galloway, Bryonna Jackson, Kimberly Jung, Edna Kabisa, Lance Moore, Joseph Nardello, Rachel Sopko, and Ceaira Walker for their assistance. This research was supported by award number R15MH079374 from the National Institute of Mental Health. The content is solely the responsibility of the author and does not necessarily represent the official views of the National Institute of Mental Health or the National Institutes of Health.
Footnotes
We also included the Center for Epidemiological Studies—Depression Scale (CES-D; Radloff, 1977) as a secondary measure of depressive symptoms. The CES-D covers a broader range of symptoms, not just anhedonic ones, and it is the scale used in all the studies reported by Brinkmann and her colleagues on dysphoria and effort. We thus included it to evaluate the unlikely possibility of scale-specific effects that might explain differences between the present study and their findings. The DASS and CES-D correlated highly (r = .81, p < .001), and the physiological effects were essentially identical: everything that was significant for the DASS was significant for the CES-D, and vice-versa. Differences between the present findings and past research thus aren’t due to using different depression scales.
Several researchers have recently suggested using the time difference (in ms) between the R point (the peak of the ECG) and the Z point (the peak of the dz/dt) as a measure of contractility (e.g., Cybulski, 2011; Meijer, Boesveldt, Elbertse, & Berendse, 2008; van Lien, Schutte, Meijer, & de Geus, 2013). This index has been called the RZ interval or the initial systolic time interval, and several studies suggest it is an effective measure of left ventricular contractility (e.g., van der Meer, Noordegraaf, Bax, Kamp, & de Vries, 1999; Wilde et al., 1981). To inform this emerging literature, we estimated the same multilevel model using RZ intervals as the outcome. The same effects appeared: RZ intervals decreased from task to baseline overall, reflecting increased sympathetic activity (b = −1.41, SE = .43, p < .001); DASS scores had a main effect on RZ intervals, reflecting less baseline sympathetic impact as depressive symptoms increased (b = 7.62, SE = 2.42, p = .002); and DASS scores moderated the effect of time on RZ intervals, reflecting larger RZ intervals (and hence less sympathetic impact) as depressive symptoms increased (b = 2.45, SE = .55, p < .001). The similar pattern and higher significance levels lend some weight to the use of RZ intervals as a complementary measure of sympathetic influence.
Some research on effort and HRV (e.g., Segerstrom & Nes, 2007) has used time-domain measures, such as the root mean square of successive differences (RMSSD) of the interbeat intervals, instead of frequency-domain measures (e.g., RSA). In our data, the results were largely the same for RMSSD, with one notable difference. At the within-person level, there was a marginal main effect of time, b = 3.42, SE = 1.82, p = .061. Unlike RSA, which had a null effect, RMSSD increased from baseline to task, reflecting stronger parasympathetic activity. At the between-person level, depressive symptoms did not predict either the RMSSD intercept (b 2.03, SE = 6.88, p = .768) or change in RMSSD from baseline to task (b = −1.66, SE = 2.36, p .483). The main effect of time—an increase in RMSSD from baseline to task—is consistent with Segerstrom’s proposal that HRV can reflect increased self-regulatory control under some circumstances (Segerstrom, Hardy, Evans, & Winters, 2012).
We can appreciate that multilevel models are unfamiliar for many motivation researchers, so we also analyzed the central PEP finding using more common reactivity scores. These analyses are more familiar and illustrate the parallels between multilevel models and traditional difference-score approaches. We created a PEP reactivity score by subtracting PEP scores during the baseline (the average of the 5 periods) from the PEP scores (averaged across the 5 periods) during the parity task. The reactivity scores didn’t correlate with the baseline scores (r = .12, p = .22), so they were not residualized with respect to the baseline (Llabre, Spitzer, Saab, Ironson, & Schneiderman, 1991). For the full sample, DASS scores correlated with baseline scores (r = .23, p = .012), which is akin to the between-person main effect of DASS scores on PEP scores in the multilevel model—PEP slowed as DASS scores increased. DASS scores also correlated with PEP reactivity scores (r = .29, p = .002), which is akin to the significant interaction between DASS scores and time (baseline vs. task) in the multilevel model. People with lower DASS scores had more strongly negative change scores, and vice versa.
We then selected the upper and lower 30% based on DASS scores, akin to the use of extreme groups in past work (e.g., Brinkmann & Gendolla, 2007, 2008), which yielded a sample of 72 people. The average reactivity score was M =−.58 (SE = .33), indicating that for the sample as whole, PEP was faster during the parity task. But PEP reactivity was faster in the low DASS group (M = −1.43, SE = .55, 95% CI = −2.56, −.30) than in the high DASS group (M = .15, SE = .36, 95% CI = −.59, .88). As the confidence intervals show, the low DASS group had PEP reactivity scores that both differed significantly from zero (reflecting significant change from the baseline) and from the high DASS group (reflecting a significant between-group difference). PEP reactivity in the high DASS group, in contrast, didn’t differ significantly from zero, reflecting a lack of effort mobilization.
References
- Antony MM, Beiling PJ, Cox BJ, Enns MW, Swinson RP. Psychometric properties of the 42-item and 21-item versions of the Depression Anxiety Stress Scales (DASS) in clinical groups and a community sample. Psychological Assessment. 1998;10:176–181. [Google Scholar]
- Berntson GG, Cacioppo JT, Quigley KS. Respiratory sinus arrhythmia: Autonomic origins, physiological mechanisms, and psychophysiological implications. Psychophysiology. 1993b;30:183–196. doi: 10.1111/j.1469-8986.1993.tb01731.x. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Lozano DL, Chen Y, Cacioppo JT. Where to Q in PEP. Psychophysiology. 2004;41:333–337. doi: 10.1111/j.1469-8986.2004.00156.x. [DOI] [PubMed] [Google Scholar]
- Brehm JW, Self EA. The intensity of motivation. Annual Review of Psychology. 1989;40:109–131. doi: 10.1146/annurev.ps.40.020189.000545. doi:10.1146/annurev.ps.40.020189.000545. [DOI] [PubMed] [Google Scholar]
- Brinkmann K, Franzen J. Depression and self-regulation: A motivational analysis and insights from effort-related cardiovascular activity. In: Gendolla GHE, Mattie Tops, Sander Koole, editors. Biobehavioral foundations of self-regulation. Springer; New York: in press. [Google Scholar]
- Brinkmann K, Franzen J. Not everyone’s heart contracts to reward: Insensitivity to varying levels of reward in dysphoria. Biological Psychology. 2013;94:263–271. doi: 10.1016/j.biopsycho.2013.07.003. [DOI] [PubMed] [Google Scholar]
- Brinkmann K, Gendolla GHE. Dysphoria and mobilization of mental effort: Effects on cardiovascular reactivity. Motivation and Emotion. 2007;31:71–82. [Google Scholar]
- Brinkmann K, Gendolla GHE. Does depression interfere with effort mobilization? Effects of dysphoria and task difficulty on cardiovascular response. Journal of Personality and Social Psychology. 2008;94:146–157. doi: 10.1037/0022-3514.94.1.146. [DOI] [PubMed] [Google Scholar]
- Brinkmann K, Grept J, Gendolla GHE. Dysphorics can control depressive mood’s informational impact on effort mobilization. Motivation and Emotion. 2012;36:232–241. [Google Scholar]
- Brinkmann K, Schüpbach L, Joye IA, Gendolla GHE. Anhedonia and effort mobilization in dysphoria: Reduced cardiovascular response to reward and punishment. International Journal of Psychophysiology. 2009;74:250–258. doi: 10.1016/j.ijpsycho.2009.09.009. [DOI] [PubMed] [Google Scholar]
- Brown TA, Chorpita BF, Korotitsch W, Barlow DH. Psychometric properties of the Depression Anxiety Stress Scales (DASS) in clinical samples. Behaviour Research and Therapy. 1997;35:79–89. doi: 10.1016/s0005-7967(96)00068-x. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Berntson GG, Binkley PF, Quigley KS, Uchino BN, Fieldstone A. Autonomic cardiac control. II. Noninvasive indices and basal response as revealed by autonomic blockades. Psychophysiology. 1994;31:586–598. doi: 10.1111/j.1469-8986.1994.tb02351.x. [DOI] [PubMed] [Google Scholar]
- Cybulski G. Ambulatory impedance cardiography: The systems and their applications. Springer; Heidelberg, Germany: 2011. [Google Scholar]
- de Geus EJC, Willemsen GHM, Klaver CHAM, van Doornen LJP. Ambulatory assessment of respiratory sinus arrhythmia and respiration rate. Biological Psychology. 1995;41:205–227. doi: 10.1016/0301-0511(95)05137-6. [DOI] [PubMed] [Google Scholar]
- Drew RC, Sinoway LI. Autonomic control of the heart. In: Robertson D, Biaggioni I, Burnstock G, Low PA, Paton JFR, editors. Primer on the autonomic nervous system. 3rd ed. Academic Press; London: 2012. pp. 177–180. [Google Scholar]
- Ernst JM, Litvack DA, Lozano DL, Cacioppo JT, Berntson GG. Impedance pneumography: Noise as signal in impedance cardiography. Psychophysiology. 1999;36:333–338. doi: 10.1017/s0048577299981003. [DOI] [PubMed] [Google Scholar]
- Gendolla GHE. On the impact of mood on behavior: An integrative theory and a review. Review of General Psychology. 2000;4:378–408. [Google Scholar]
- Gendolla GHE, Abele AE, Krüsken J. The informational impact of mood on effort mobilization: A study of cardiovascular and electrodermal responses. Emotion. 2001;1:12–24. doi: 10.1037/1528-3542.1.1.12. [DOI] [PubMed] [Google Scholar]
- Gendolla GE, Brinkmann K, Silvestrini N. Gloomy and lazy? On the impact of mood and depressive symptoms on effort-related cardiovascular response. In: Wright RA, Gendolla GHE, editors. How motivation affects cardiovascular response: Mechanisms and applications. American Psychological Association; Washington, DC: 2012. pp. 139–155. [Google Scholar]
- Graziano P, Derefinko K. Cardiac vagal control and children’s adaptive functioning: A meta-analysis. Biological Psychology. 2013;94:22–37. doi: 10.1016/j.biopsycho.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman P, Taylor EW. Toward understanding respiratory sinus arrhythmia: Relations to cardiac vagal tone, evolution, and biobehavioral functions. Biological Psychology. 2007;74:263–285. doi: 10.1016/j.biopsycho.2005.11.014. [DOI] [PubMed] [Google Scholar]
- Houtveen JH, Groot PF, de Geus EJ. Validation of the thoracic impedance derived respiratory signal using multilevel analysis. International Journal of Psychophysiology. 2006;59:97–106. doi: 10.1016/j.ijpsycho.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Kelsey RM. Beta-adrenergic cardiovascular reactivity and adaptation to stress: The cardiac pre-ejection period as an index of effort. In: Wright RA, Gendolla GHE, editors. How motivation affects cardiovascular response: Mechanisms and applications. American Psychological Association; Washington, DC: 2012. pp. 43–60. [Google Scholar]
- Kelsey RM, Reiff S, Wiens S, Schneider TR, Mezzacappa ES, Guethlein W. The ensemble-averaged impedance cardiogram: An evaluation of scoring methods and interrater reliability. Psychophysiology. 1998;35:337–340. doi: 10.1017/s0048577298001310. [DOI] [PubMed] [Google Scholar]
- Kemp AH, Quintana DS, Gray MA, Felmingham KL, Brown K, Gatt JM. Impact of depression and antidepressant treatment on heart rate variability: A review and meta-analysis. Biological Psychiatry. 2010;67:1067–1074. doi: 10.1016/j.biopsych.2009.12.012. [DOI] [PubMed] [Google Scholar]
- Kristjansson SD, Kircher JC, Webb AK. Multilevel models for repeated measures designs in psychophysiology: An introduction to growth curve modeling. Psychophysiology. 2007;44:728–736. doi: 10.1111/j.1469-8986.2007.00544.x. [DOI] [PubMed] [Google Scholar]
- Light KC, Kothandapani RV, Allen MT. Enhanced cardiovascular and catecholamine responses in women with depressive symptoms. International Journal of Psychophysiology. 1998;28:157–166. doi: 10.1016/s0167-8760(97)00093-7. [DOI] [PubMed] [Google Scholar]
- Llabre MM, Spitzer SB, Saab PG, Ironson GH, Schneiderman N. The reliability and specificity of delta versus residualized change as measure of cardiovascular reactivity to behavioral challenges. Psychophysiology. 1991;28:701–711. doi: 10.1111/j.1469-8986.1991.tb01017.x. [DOI] [PubMed] [Google Scholar]
- Llabre MM, Spitzer S, Siegel S, Saab PG, Schneiderman N. Applying latent growth curve modeling to the investigation of individual differences in cardiovascular recovery from stress. Psychosomatic Medicine. 2004;66:29–41. doi: 10.1097/01.psy.0000107886.51781.9c. [DOI] [PubMed] [Google Scholar]
- Lovibond PF. Long-term stability of depression, anxiety, and stress syndromes. Journal of Abnormal Psychology. 1998;107:520–526. doi: 10.1037//0021-843x.107.3.520. [DOI] [PubMed] [Google Scholar]
- Lovibond PF, Lovibond SH. The structure of negative emotional states: Comparison of the Depression Anxiety Stress Scales (DASS) with the Beck Depression and Anxiety Inventories. Behaviour Research and Therapy. 1995;33:335–343. doi: 10.1016/0005-7967(94)00075-u. [DOI] [PubMed] [Google Scholar]
- Lozano DL, Norman G, Knox D, Wood BL, Miller BD, Emery CF, Berntson GG. Where to B in dZ/dt. Psychophysiology. 2007;44:113–119. doi: 10.1111/j.1469-8986.2006.00468.x. [DOI] [PubMed] [Google Scholar]
- Meijer JH, Boesveldt S, Elbertse E, Berendse HW. Method to measure autonomic control of cardiac function using time interval parameters from impedance cardiography. Physiological Measurement. 2008;29:383–391. doi: 10.1088/0967-3334/29/6/S32. doi:10.1088/0967-3334/29/6/S32. [DOI] [PubMed] [Google Scholar]
- Mohrman DE, Heller LJ. Cardiovascular physiology. 7th ed. McGraw Hill Medical; New York: 2010. [Google Scholar]
- Obrist PA, Light KC, James SA, Strogatz DS. Cardiovascular responses to stress: I. Measures of myocardial response and relationship to high resting systolic pressure and parental hypertension. Psychophysiology. 1987;24:65–78. doi: 10.1111/j.1469-8986.1987.tb01864.x. [DOI] [PubMed] [Google Scholar]
- Phillips AC. Blunted cardiovascular reactivity relates to depression, obesity, and self-reported health. Biological Psychology. 2011;86:106–113. doi: 10.1016/j.biopsycho.2010.03.016. [DOI] [PubMed] [Google Scholar]
- Phillips AC, Hughes BM. Cardiovascular reactivity at a crossroads: Where are we now? Biological Psychology. 2011;86:95–97. doi: 10.1016/j.biopsycho.2010.03.003. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Richter M. Pay attention to your manipulation checks! Reward impact on cardiac reactivity is moderated by task context. Biological Psychology. 2010;84:279–289. doi: 10.1016/j.biopsycho.2010.02.014. [DOI] [PubMed] [Google Scholar]
- Richter M. A closer look into the multi-layer structure of motivational intensity theory. Social and Personality Psychology Compass. 2013;7:1–12. [Google Scholar]
- Richter M, Friedrich A, Gendolla GHE. Task difficulty effects on cardiac activity. Psychophysiology. 2008;45:869–875. doi: 10.1111/j.1469-8986.2008.00688.x. [DOI] [PubMed] [Google Scholar]
- Richter M, Gendolla GHE. Incentive effects on cardiovascular reactivity in active coping with unclear task difficulty. International Journal of Psychophysiology. 2006;61:216–225. doi: 10.1016/j.ijpsycho.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Richter M, Gendolla GHE. Incentive value, unclear task difficulty, and cardiovascular reactivity in active coping. International Journal of Psychophysiology. 2007;63:294–301. doi: 10.1016/j.ijpsycho.2006.12.002. [DOI] [PubMed] [Google Scholar]
- Richter M, Gendolla GHE. The heart contracts to reward: Monetary incentives and pre-ejection period. Psychophysiology. 2009a;46:451–457. doi: 10.1111/j.1469-8986.2009.00795.x. [DOI] [PubMed] [Google Scholar]
- Richter M, Gendolla GHE. Mood impact on cardiovascular reactivity when task difficulty is unclear. Motivation and Emotion. 2009b;33:239–248. [Google Scholar]
- Rottenberg J. Cardiac vagal control in depression: A critical analysis. Biological Psychology. 2007;74:200–211. doi: 10.1016/j.biopsycho.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Rottenberg J, Clift A, Bolden S, Salomon K. RSA fluctuation in major depressive disorder. Psychophysiology. 2007;44:450–458. doi: 10.1111/j.1469-8986.2007.00509.x. [DOI] [PubMed] [Google Scholar]
- Rottenberg J, Salomon K, Gross JJ, Gotlib IH. Vagal withdrawal to a sad film predicts subsequent recovery from depression. Psychophysiology. 2005;42:277–281. doi: 10.1111/j.1469-8986.2005.00289.x. [DOI] [PubMed] [Google Scholar]
- Salomon K, Clift A, Karlsdóttir M, Rottenberg J. Major depressive disorder is associated with attenuated cardiovascular reactivity and impaired recovery among those free of cardiovascular disease. Health Psychology. 2009;28:157–165. doi: 10.1037/a0013001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schächinger H, Weinbacher M, Kiss A, Ritz R, Langewitz W. Cardiovascular indices of peripheral and central sympathetic activation. Psychosomatic Medicine. 2001;63:788–796. doi: 10.1097/00006842-200109000-00012. [DOI] [PubMed] [Google Scholar]
- Schwerdtfeger A, Rosenkaimer AK. Depressive symptoms and attenuated physiological reactivity to laboratory stressors. Biological Psychology. 2011;87:430–438. doi: 10.1016/j.biopsycho.2011.05.009. [DOI] [PubMed] [Google Scholar]
- Segerstrom SC, Hardy JK, Evans DR, Winters NF. Pause and plan: Self-regulation and the heart. In: Wright RA, Gendolla GHE, editors. How motivation affects cardiovascular response: Mechanisms and applications. American Psychological Association; Washington, DC: 2012. pp. 181–198. [Google Scholar]
- Segerstrom SC, Nes LS. Heart rate variability reflects self-regulatory strength, effort, and fatigue. Psychological Science. 2007;18:275–281. doi: 10.1111/j.1467-9280.2007.01888.x. [DOI] [PubMed] [Google Scholar]
- Sherwood A, Allen MT, Fahrenberg J, Kelsey RM, Lovallo WR, van Doornen LJ. Methodological guidelines for impedance cardiography. Psychophysiology. 1990;27:1–23. doi: 10.1111/j.1469-8986.1990.tb02171.x. [DOI] [PubMed] [Google Scholar]
- Silvia PJ. Mirrors, masks, and motivation: Implicit and explicit self-focused attention influence effort-related cardiovascular reactivity. Biological Psychology. 2012;90:192–201. doi: 10.1016/j.biopsycho.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvia PJ, Eddington KM, Beaty RE, Nusbaum EC, Kwapil TR. Gritty people try harder: Grit and effort-related cardiac autonomic activity during an active coping challenge. International Journal of Psychophysiology. 2013;88:200–205. doi: 10.1016/j.ijpsycho.2013.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvia PJ, Jones HC, Kelly CS, Zibaie A. Masked first name priming increases effort-related cardiovascular reactivity. International Journal of Psychophysiology. 2011a;80:210–216. doi: 10.1016/j.ijpsycho.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvia PJ, Jones HC, Kelly CS, Zibaie A. Trait self-focused attention, task difficulty, and effort-related cardiovascular reactivity. International Journal of Psychophysiology. 2011b;79:335–340. doi: 10.1016/j.ijpsycho.2010.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvia PJ, Kelly CS, Zibaie A, Nardello JL, Moore LC. Trait self-focused attention increases sensitivity to nonconscious primes: Evidence from effort-related cardiovascular reactivity. International Journal of Psychophysiology. 2013;88:143–148. doi: 10.1016/j.ijpsycho.2013.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvia PJ, McCord DM, Gendolla GHE. Self-focused attention, performance expectancies, and the intensity of effort: Do people try harder for harder goals? Motivation and Emotion. 2010;34:363–370. [Google Scholar]
- Silvia PJ, Moore LC, Nardello JL. Trying and quitting: How self-focused attention influences effort during difficult and impossible tasks. Self and Identity. in press. [Google Scholar]
- Silvia PJ, Phillips AG. Self-awareness without awareness? Implicit self-focused attention and behavioral self-regulation. Self and Identity. 2013;12:114–127. doi: 10.1080/15298868.2011.639550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer JD, Willett JB. Applied longitudinal data analysis: Modeling change and event occurrence. Oxford University Press; New York: 2003. [Google Scholar]
- van der Meer BJM, Noordegraaf AV, Bax JJ, Kamp O, de Vries PMJM. Non-invasive evaluation of left ventricular function by means of impedance cardiography. Acta Anaesthesiologica Scandinavica. 1999;43:130–134. doi: 10.1034/j.1399-6576.1999.430203.x. [DOI] [PubMed] [Google Scholar]
- van Lien R, Schutte NM, Meijer JH, de Geus EJC. Estimated prejection period (PEP) based on the detection of the R-wave and dZ/dt-min peaks does not adequately reflect the actual PEP across a wide range of laboratory and ambulatory conditions. International Journal of Psychophysiology. 2013;87:60–69. doi: 10.1016/j.ijpsycho.2012.11.001. [DOI] [PubMed] [Google Scholar]
- Wilde SW, Miles DS, Durbin RJ, Sawka MN, Suryaprasad AG, Gotshall RW, Glaser RM. Evaluation of myocardial performance during wheelchair ergometer exercise. American Journal of Physical Medicine. 1981;60:277–291. [PubMed] [Google Scholar]
- Wright RA. Brehm’s theory of motivation as a model of effort and cardiovascular response. In: Gollwitzer PM, Bargh JA, editors. The psychology of action: Linking cognition and motivation to behavior. Guilford; New York: 1996. pp. 424–453. [Google Scholar]
- Wright RA. Ability perception and cardiovascular response to behavioral challenge. In: Kofta M, Weary G, Sedek G, editors. Personal control in action: Cognitive and motivational mechanisms. Plenum; New York: 1998. pp. 197–232. [Google Scholar]
- Wright RA. Refining the prediction of effort: Brehm’s distinction between potential motivation and motivation intensity. Social and Personality Psychology Compass. 2008;2:682–701. [Google Scholar]
- Wright RA, Dill JC. Blood pressure responses and incentive appraisals as a function of perceived ability and objective task demand. Psychophysiology. 1993;30:152–160. doi: 10.1111/j.1469-8986.1993.tb01728.x. [DOI] [PubMed] [Google Scholar]
- Wright RA, Gendolla GHE. How motivation affects cardiovascular response: Mechanisms and applications. American Psychological Association; Washington, DC: 2012. [Google Scholar]
- Wright RA, Killebrew K, Pimpalapure D. Cardiovascular incentive effects where a challenge is unfixed: Demonstrations involving social evaluation, evaluator status, and monetary reward. Psychophysiology. 2002;39:188–197. doi: 10.1017/S0048577202011137. [DOI] [PubMed] [Google Scholar]
- Wright RA, Stewart CC. Multifaceted effects of fatigue on effort and associated cardiovascular responses. In: Wright RA, Gendolla GHE, editors. How motivation affects cardiovascular response: Mechanisms and applications. American Psychological Association; Washington, DC: 2012. pp. 199–218. [Google Scholar]