Abstract
There is a growing need in drug discovery and basic research to measure multiple second messenger components of cell signaling pathways in real time and in relevant tissues and cell types. Many G-protein coupled receptors activate the heterotrimeric protein, Gq, which in turn activates phospholipase C (PLC; Figure 1). PLC cleaves Phosphatidylinositol 4,5-bisphosphate (PIP2) to produce two second messengers: diacylglycerol (DAG), which remains in the plasma membrane, and inositol triphosphate (IP3), which diffuses through the cytosol to release stores of intracellular calcium ions (Ca2+). Our goal was to create a series of multiplex sensors that would make it possible to simultaneously measure two different components of the Gq pathway in living cells. Here we describe new fluorescent sensors for DAG and PIP2 that produce robust changes in green or red fluorescence, and can be combined with one another, or with existing Ca2+ sensors, in a live cell assay. These assays can detect multiple components of Gq signaling, simultaneously in real time, on standard fluorescent plate readers or live cell imaging systems.
Introduction
Cell signaling involves the concerted activity of multiple second messenger pathways. Figure 1 is a simplistic diagram of just Gq signaling, and it illustrates how many different proteins and second messengers are involved in parallel pathways. It is the balance of these different signaling components, coordinated in both space and time, that ultimately dictate the response of the cell. While this is well understood in theory, the practice of measuring signaling is often reduced to two time points - before and after drug - and to a single second messenger. When kinetic measurements of signaling are possible, a new level of precision and insight guide new experiments and optimized assays. In the cases that it has been possible to image multiple components of a signaling pathway in the same cells (1–4), the interplay between the different components has provided new insights into the biological system and the downstream consequences of a drug’s actions.
Figure 1.

A simple diagram of GPCR signaling through Gq. The activated receptor catalyses the activation of the heterotrimeric Gq protein. The Gqα activates phospholipase C (PLC) which cleaves phosphatidylinositol 4,5-bisphosphate to produce both diacylglycerol and inositol 1,4,5-trisphosphate (IP3). The IP3 triggers the release of Ca2+ from intracellular stores, and the combination of raised intracellular Ca2+ and DAG activates conventional PKC enzymes with a multitude of down stream targets. The goal of this work was to create independent sensors for Diacylglycerol and PIP2 that could be multiplexed with one another, as well as existing Ca2+ sensors, to detect the concerted pattern of Gq signaling activity. No one sensor in isolation is capable of unambiguously detecting Gq signaling since other pathways can produce elevated levels of DAG, PIP2, or Ca2+.
Multiplex sensors capable of simultaneously detecting different signaling components are particularly important to advancing our search for drugs that interact with G-protein coupled receptors. This importance can be appreciated in the context of either the traditional view of GPCR signaling or in the framework of the more recent agonist-bias signaling at particular GPCRs.
Traditional models of GPCR signaling involve activated G protein α and β subunits as the crucial first components of signaling which then act upon effectors. Even in this relatively simple model, there are multiple effectors, and multiple second messengers, all acting in concert. Most, if not all, of these second messengers can be influenced by other signaling pathways. Even in this model, multiplex sensors are necessary to decode the pathway or pathways involved in the response to the activation of a particular GPCR. For example, Gq signaling produces a rise in intracellular Ca2+, but many other pathways do as well. To unambiguously identify Gq signaling, it is necessary to measure other components as well.
In the more recent model of agonist-biased signaling, an even more pressing case can be made for multiplex sensor systems (5–9). A wide variety of evidence, including recent structural studies, has culminated in a multi-state model of GPCR activation in which different agonists stabilize a particular receptor in a conformation that activates a unique pattern of intracellular signaling. In simple terms, different agonists can produce different levels of Gα, Gβ, and arrestin signaling. This selectivity is particularly important in the scenario where a receptor can access both an important activity, such as analgesia, via one pathway, and unintended consequences, side effects, via another (10). Multiplex sensors that can simultaneously measure multiple pathways will be critical to assessing the biological relevance of a particular drug (7).
What are the design criteria for optimal multiplex sensors? First, they need to work in living cells and provide kinetic data for each signaling pathway. This means they need to provide strong signals that can be sampled at the Nyquist frequency, which for cellular signaling events (200 ms - 5 s) can be up to 10 Hz. Second, each sensor needs to consume as little of the visible spectrum as possible so that there is minimal crosstalk with other sensors. Finally, each sensor has to specifically detect the second messenger at physiologically relevant concentrations.
Fluorescent protein-based sensors meet many of the design criteria: they work in living cells, they produce strong signals that can be sampled repeatedly and quickly, and the protein domains they carry have evolved to specifically detect a particular second messenger (1). However, early sensors based upon Förster Resonance Energy Transfer (FRET) between two different fluorescent proteins, rarely produce the sort of robust signals necessary for automated detection. Furthermore, the broad absorption bands of the donor and acceptor fluorophores consume most of the visible spectrum (11, 12).
More recently, a new generation of fluorescent protein sensors has been developed that only uses one fluorescent protein, produces large changes in fluorescence, and has the potential for multiplexing. Many of these new sensors carry a single, circularly permuted fluorescent protein that converts analyte binding into changes in fluorescence intensity. The green fluorescent G-CaMP Ca2+ sensors (13–15), the red R-GECO Ca2+ sensors (16), the green ElectricPk voltage sensor (17), the green cGMP sensor (18), as well as our own prototype sensors for DAG use this approach (19).
Many drugs act at G-protein coupled receptors on the cell surface. Some of these receptors couple to the heterotrimeric protein, Gq, which activates phospholipase C (PLC). PLC in turn cleaves PIP2 to produce two second messengers: diacylglycerol (DAG), which remains in the plasma membrane, and inositol triphosphate (IP3), which diffuses through the cytosol to release stores of intracellular calcium ions (Ca2+). This coordinated increase, in both DAG and cytosolic Ca2+, triggers the activation of conventional isoforms of protein kinase C (cPKC) which then phosphorylate many different targets. To explore the potential of multiplex sensors for the Gq pathway, particularly in the context of drug discovery and laboratory automation, we improved our green fluorescent DAG sensors, created a new, robust red fluorescent PIP2 sensor, and multiplexed these with one another or with existing Green and Red Ca2+ sensors.
Methods and Materials
PCR amplified fragments of the PKCδ coding region and a circularly permuted GFP from G-GECO1 (16) were combined and cloned into a modified version of the mammalian expression vector pcDNA3.1 using the In-Fusion Cloning system (Clontech Laboratories Inc, Mountain View, CA). The pcDNA3.1 vector was obtained from Life Technologies (Grand Island, NY). Sixty four unique candidate diacylglycerol sensors resulted from combining fragments of PKCδ with cpEGFP. Thirty two constructs contained the cpEGFP insert in the full length PKCδ, an additional 32 constructs contained eGFP in a truncated PKCδ where the C2 domain was deleted.
HEK 293 cells (20) were cultured in Eagle’s minimal essential medium (EMEM) supplemented with 10% fetal bovine serum and Penicillin-Streptomycin at 37 °C in 5% CO2. The cells and (EMEM) were purchased from ATCC (Manassas, VA). Prior to cell seeding, 96-well glass-bottom plates were coated with Poly-D-Lysine. Cells were seeded on the plates, transfected using Lipofectamine 2000 Transfection Reagent according to the manufacturer’s protocol, and incubated for 24–48 hours at 37 °C in 5% CO2. 60 ng of sensor DNA was co-transfected with 40 ng of human M1 muscarinic acetylcholine receptor per well. Pen-Strep liquid and Lipofectamine 2000 were obtained from Life Technologies (Grand Island, NY). Poly-D-Lysine was purchased from Fisher Scientific (Pittsburg, PA).
EMEM culture medium was replaced with 1× Dulbecco’s phosphate-buffered saline (DPBS) prior to screening transfected cells for fluorescence. A Zeiss Axiovert S100TV inverted microscope equipped with computer controlled excitation/emission filter wheels, shutters, and a Qimaging Retiga Exi CCD camera (Surrey, BC Canada) was used to image cells at 25 °C using the 10× objective lens (N.A. 0.3). A combination of 480 ± 20 nm excitation and 535 ± 25 nm emission filters were used resolve the green fluorescence from the DAG sensors, and 572 ± 20 nm and 630 ± 30 nm filters were used to collect the R-GECO signal. Cells were analyzed for increases or decreases in fluorescence intensity upon addition of Carbachol, Phorbol 12,13-dibutyrate (PdBU), Dimethyl sulfoxide (DMSO) or Ionomycin. To analyze the image stacks, background fluorescence was defined as a region of the image that contained no cells. The average value of this region was subtracted frame by frame from the measurements of the mean pixel values of the fluorescent cells. Fluorescence intensity data was plotted and analyzed with IGOR (Wavemetrics, Oswego Ore.).
For transient expression and screening in an automated fluorescence plate reader, HEK 293T cells were cultured in Corning Co-Star Polystyrene 96 well plates coated with Poly-D-Lysine. Seventy μl of a 25 ug/ml Poly-D-Lysine solution was added to each well for 1 hr, and then the well was rinsed 1× with sterile DH2O and dried (coating concentration ~ 5 μg/cm2). HEK293T cells were plated at 35,0000 cells/well in 100 μl growth medium per well without antibiotics so that the cells would be 90–95% confluent at the time of transfection (approximately 24 hours later). For each transfection (i.e. one well in a 96-well plate), 160 ng of plasmid DNA (120 ng sensor+ 40ng receptor) was diluted in 25 μl of Opti-MEM, 0.48 ul of lipofectamine 2000 was diluted in 25 μl of Opti-MEM, and these were then mixed and added to the cells. Cells were incubated in this mixture for 4 to 6 hours, and then the mixture was replaced with fresh medium. Prior to scanning a plate on the Biotek Synergy Mx, EMEM culture medium is replaced with 250 μl of 1× DPBS per well. Plates were read at 25°C, using monochromators set to 488/20 nm excitation and 530/20 nm emission to resolve the green fluorescence from the DAG sensor.
Results
To reliably detect the activation of the PLC pathway, we created a series of genetically encoded, fluorescent DAG sensors. Sixty four candidates were produced that fused a circularly permuted green fluorescent protein to PKCδ, which is a novel isoform that only responds to DAG because only the C1 domain is functional (21, 22). Two robust prototype sensors, previously described, were recovered from this initial effort (19). In both of these sensors, the circularly permuted green fluorescent protein and the linker are positioned between the pseudosubstrate and the C1 domains of PKCδ. Surprisingly, one sensor increases fluorescence as a result of activation (Upward DAG), while an insertion only 6 amino acids away produced a sensor that decreases fluorescence as a result of activation (Downward DAG). To our knowledge, this is the first example of small change in the position of the fluorescent protein producing an inversion of the signal produced by the sensor. However, it is well known that minor adjustments in the linkers interconnecting the circularly permuted fluorescent protein and the analyte-sensing domains can have a large impact on the amplitude of the fluorescence change (13, 14). To optimize these prototype sensors, we created an additional 156 variants of the original Upward and Downward DAG sensors. This produced the Upward DAG2 sensor, which produces a much larger response than the initial Upward DAG sensor, while it continues to display very similar response kinetics. These sensors can be readily co-expressed with the red fluorescent R-GECO1 Ca2+ sensors to simultaneously measure both second messengers in living cells (Figure 2, A). Both the onset of the Ca2+ response and the return to baseline is considerably quicker than the the DAG response, which is consistent with previous measurements (23, 24).
Figure 2.
The green fluorescent sensors Upward DAG2 and Downward DAG can be co-expressed with the red fluorescent R-GECO1 to simultaneously measure Ca2+ and DAG signaling in living cells. The upper panel compares the Upward DAG2 sensor signal with the R-GECO1 response in the same cell. The lower panel compares the Downward DAG sensor with R-GECO1 in three different cells (identified by symbol). The green fluorescence (mean pixel intensity) is plotted in green using the left axis (arbitrary units) and the right axis represents red fluorescence. The effect of DMSO on the DAG sensors is negligible at final concentrations of 0.1 to 1%, but detectable at 2% or greater (B).
Many compound libraries are carried by DMSO, a vehicle that can cause artifacts in live cell assays. To test the effects of DMSO on the DAG assay, DMSO of different concentrations was added to the culture, followed later by carbachol to evoke the maximal sensor response (Figure 2, B). At moderate final concentrations of 0.1 to 1%, the DMSO produced no effect, while at higher concentrations artifactual, DMSO-triggered changes in fluorescence did occur.
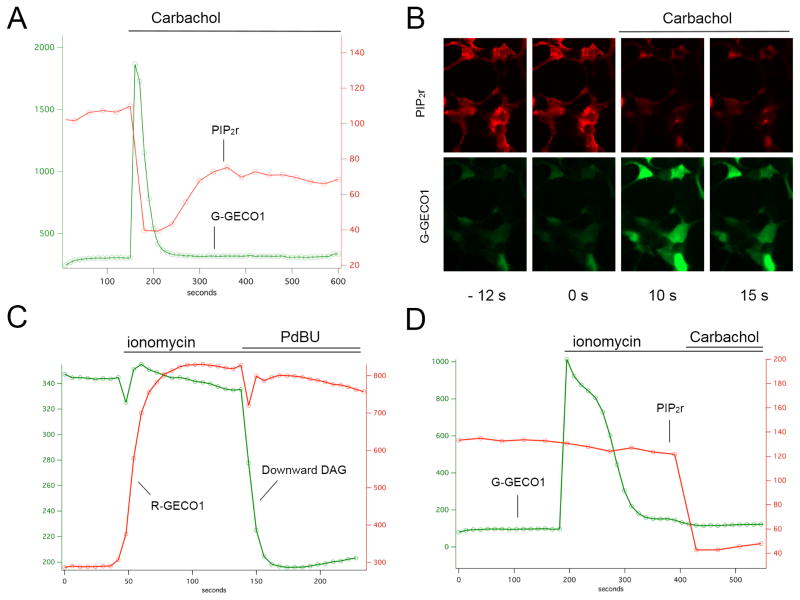
PLC hydrolyses PIP2 to produce both DAG and IP3. To independently check the fidelity and kinetics of the DAG sensors, we created a red fluorescent PIP2 sensor by fusing the pleckstrin homology (PH) domain of PLCδ to two different components of the recently described dimerization-dependent red fluorescent proteins (25). Previous work has shown that the translocation of the PLCδ PH domain can be used to measure PIP2 turnover (26), and if the PH domains carry FRET pairs of fluorescent proteins a small change in FRET occurs when PLC is activated (27). To create a more robust sensor that does not involve FRET, and which produces a larger signal with a single fluorescent protein, we fused the PH domain to each member of the ddRFP pair. One pair of constructs produced a particularly strong red fluorescent signal at the membrane that rapidly disappeared with M1 receptor activation (Figure 3A). This red fluorescent PIP2 sensor PIP2r was co-expressed with the green fluorescent G-GECO1 Ca2+ sensor (16) and stimulation of the M1 receptor produced a rapid, simultaneous rise in Ca2+ and fall in PIP2 levels. Changes in Ca2+ can have profound effects on many cellular processes. To explore the relationship between intracellular Ca2+ levels and the signals produced by our DAG and PIP2 sensors, we first raised Ca2+ levels by adding the ionophore ionomycin, and then activated the DAG sensors with PdBU and the PIP2r sensors with M1 receptor activation. Raising intracellular Ca2+ had no apparent effect on the DAG and PIP2 levels (Figure 3, C & D).
Figure 3.
Multiplexing DAG, PIP2, and Ca2+ sensors. The red PIP2r sensor was co-expressed with the green G-GECO1 Ca2+ sensor and the M1 receptor. Carbachol addition triggered a simultaneous increase in green fluorescence and decrease in red fluorescence ( A & B). To test for interactions between the Ca2+ increase and DAG (C) or PIP2r (D) sensors, ionomycin was added to the culture, followed later by carbachol or PdBU.
Co-expression of the Upward DAG2 or Downward DAG2 sensor with the PIP2r sensor provides a new view of both the substrate and product ofPLC (Figure 4). To our knowledge, this is the first time that genetically encoded biosensors have been used to simultaneously measure substrate and product. M1 receptor activation produced a remarkable change in the intensity and distribution of both probes. As expected, the PIP2r sensor rapidly leaves the membrane and looses fluorescence while the Upward DAG sensor translocates to the membrane and increases fluorescence (Figure 4, A). The onset of the response of the Upward and Downward DAG2 sensors, as well as PIP2r, is kinetically quite similar. However the return to baseline is considerably slower for the Downward DAG2 and PIP2r sensors (Figure 4, B). Because this return to baseline varies depending upon our sampling rate, our interpretation is that the apparent return to baseline for Upward DAG2 is artificially accelerated by photobleaching, and similarly prolonged in the cases of Downward DAG2 and PIP2r.
Figure 4.
The PIP2r and DAG sensors can be co-expressed and measured simultaneously. Stimulation of phospholipase C cleaves PIP2 and produces DAG, which is clearly seen in living cells as the red fluorescence of the PIP2r vanishes and the Upward DAG2 sensor increases in fluorescence (A). This is reproducible from cell to cell (B, upper panel). The apparent return to baseline for the Upward DAG2 sensor is considerably faster than the Downward DAG2 or PIP2r sensors, which may be caused photobleaching during the experiment.
White and colleagues have reported that ATP acting at the P2Y11 receptor produces inositol phosphate turnover and transient Ca2+ signaling consistent with Gq signaling, while UTP acting at the same receptor only triggers a Ca2+ response (28). To explore whether multiplex sensors could be used to detect this distinct signaling pattern, we expressed the human P2Y11 receptor with combinations of the Downward DAG2, or Upward DAG2, and R-GECO1 sensors. In HEK 293 cells, both ATP and UTP triggered a Ca2+ response that was identical in terms of kinetics (Figure 5). The Upward and Downward DAG2 sensors, however, revealed that the ATP triggers signaling via the phospholipase C pathway, while the UTP is causing a Ca2+ transient in a very different way. This UTP effect could be seen in cells that expressed only the sensor, without the P2Y11 receptor, so these results are likely to be due to the action of UTP on a receptor intrinsic to this cell line, unlike what White and colleagues saw with a different cell line (28).
Figure 5.
In HEK cells expressing the human P2Y11 receptor, the addition of ATP or UTP produces a transient increase in Ca2+ that is consistent with receptor activation. However the simultaneous recording of the Upward or Downward DAG2 sensors reveals that the ATP is activating the PLC pathway, while UTP is producing a Ca2+ transient through a different pathway.
Protein-based, fluorescent biosensors have often worked at the microscope, under exacting experimental control, and failed to make an impact on the field of laboratory automation and screening. To test whether the fluorescent DAG sensors described here would be suitable for routine applications and automated screening, we co-expressed the M1 or P2Y11 receptors with the Downward DAG2 sensor in HEK293T cells plated on a 96 well, Corning Co-Star Polystyrene plate. Media was replaced with PBS, and the fluorescence of each well before and after the addition of drug was measured using a standard plate reader. The change in fluorescence was measured for addition of vehicle alone as well as vehicle carrying carbachol or ATP. Using only the signal provided by Downward DAG2, we were able to obtain Z′ values (29) of 0.6 or greater (Figure 6, A).
Figure 6.
The DAG sensors described here are compatible with automated drug discovery. The Downward DAG2 sensor co-expressed with the M1 or P2Y11 receptor produces a consistent, reproducible signal (Z′ > 0.6) on a standard fluorescence plate reader (A). Multiplexing the DAG sensors with R-GECO produces a two dimensional surface on which the negative control wells and positive carbachol responses are unambiguously separated (B).
Multiplex sensors offer the opportunity to improve an assay by making multiple, simultaneous, independent measurements. When both the green and red fluorescence measurements were captured from wells of cells expressing both the R-GECO1 Ca2+ sensor and the Downward DAG2 sensor, it was possible to plot the response to M1 receptor activation in terms of both sensors. This reveals that there is a strong correlation between the amplitude of the two signals, and even more importantly, that the two independent signals can be used to increase the stringency of the assay, and separation between stimulated and unstimulated cells (Figure 6, B).
Discussion
The DAG sensors described here produce large changes in fluorescence that can be readily detected even on simple fluorescent plate readers. Because the sensors are based upon single fluorescent proteins, they can be readily multiplexed with other fluorescent protein based sensors such as the R-GECO series (30), or the PIP2 r sensor described here, as well as with fluorescent dyes. Such multiplexing can improve the quality of the information produced in a screen in several ways. First, the simultaneous detection of multiple components of a signaling pathway provides an unambiguous read-out for a particular pathway. Second, detection of two different signals can be used to improve assay performance/reliability. Finally, the use of multiplex sensors such as these have the potential to provide new views of agonist-biased signaling by providing relative ratios of the activity of different signaling components (7).
The multiplex sensors described here offer new opportunities for live cell assays by producing large, reproducible changes in fluorescence that can be detected on standard fluorescence plate readers used in laboratory automation. These live cell assays require no additional reagents, cell lysis, or complex liquid handling steps. The results obtained here (Figure 6) indicate that reasonable Z′ values can be reliably obtained from transiently transfected HEK293 cells. It is quite likely that even better values can be obtained by using stable cell lines expressing uniform levels of the sensor. Since this sensor system is genetically encoded, and carried by relatively short coding regions of DNA, it should be possible to deliver the sensor using a variety of viral transduction strategies or the BacMam system (31). Indeed transduction or transgenic expression with tissue or cell type specific promoters can deliver these sensors to appropriate cell types so that screening can be done in the most relevant cellular contexts. Finally, while Z′ is the appropriate metric for single sensors measuring single analytes, the multiplex sensors here introduce multiple, independent measurements where the separation of background and signal occurs in multidimensional space.
While our experiments demonstrate that these fluorescent protein-based sensors are ready for routine use on standard equipment, it is quite likely that even better signals will be obtained with plate readers that can measure the response of the sensors in every well over time. The advent of multiplex sensors for both Ca2+ and cAMP for example (2, 4), shows that cells can produce anti-phase, cyclic patterns of signaling that can only be detected by collecting the responses of the two sensors over time. Similarly, in the experiments described here, the Ca2+ and DAG/PIP2 responses are quite different, with different rates of onset and return to baseline. The only way to capture these interesting and biologically relevant patterns of signaling in microplate format, is to measure multiple signals over several time points at 0.1 to 5 Hz, making the Molecular Devices Fluorescent Imaging Plate Reader (FLIPR) and Hamamatsu FDSS (32, 33) ideal instruments for these assays.
The multiplex sensors described here provide just a glimpse of the near future. They focus on the Gqα limb of GPCR signaling, and they can be co-expressed with a wide variety of Gq coupled GPCRs in a screening environment. Although they could potentially be used to detect the activation of other GPCRs by co-expressing hybrid Gα subunits (34), this would be quite artificial. A better solution would be new, analogous multiplex sensor systems for other G proteins such as Gi and Gs. Beyond detecting Gα activity, new multiplex sensors capable of resolving Gβ and/or Arrestin signaling would provide new insights into agonist-biased signaling (7). Finally, while the signals generated by the sensors described here are robust enough for use on standard fluorescence plate readers, the rapid evolution of ever better signals in the analogous Ca2+ sensors (14, 30) indicates that even better multiplex sensors are certain to become available in the very near future.
Acknowledgments
This work was supported by the NIMH - National Institue of Mental Health (1R43MH096670-01A1), and The Montana SBIR Matching Funds Program (MSMFP) 12-50-RC SBIR-006 to A.M.Q. The authors thank Drs. Cathy Berlot and Tom Hynes for experimental advice and Dr. Bryan Roth, Director of the NIMH Psychoactive Drug Screening Program, for his advice and encouragement. Many thanks to Drs. Flori Sassano and Xi-Ping Huang in the Roth Lab at UNC for advice on cell culture and HTS. We are grateful to Dr. Robert Campbell for generously sharing his GECO Ca2+ sensors and constructs. The engineers at Autopilot in Bozeman, Montana contributed expertise in the design and production of specialized plate reader components.
References
- 1.Depry C, Mehta S, Zhang J. Multiplexed visualization of dynamic signaling networks using genetically encoded fluorescent protein-based biosensors. Pflugers Arch - Eur J Physiol. 2012:1–9. doi: 10.1007/s00424-012-1175-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aye-Han NN, Allen MD, Ni Q, Zhang J. Parallel tracking of cAMP and PKA signaling dynamics in living cells with FRET-based fluorescent biosensors. Mol Biosyst. 2012;8:1435–1440. doi: 10.1039/c2mb05514g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zaccolo M, Pozzan T. cAMP and Ca2+ interplay: a matter of oscillation patterns. Trends in Neurosciences. 2003;26:53–55. doi: 10.1016/s0166-2236(02)00017-6. [DOI] [PubMed] [Google Scholar]
- 4.Borodinsky LN, Spitzer NC. Second Messenger Pas de Deux: The Coordinated Dance Between Calcium and cAMP. Science’s STKE. 2006;2006:pe22–pe22. doi: 10.1126/stke.3362006pe22. [DOI] [PubMed] [Google Scholar]
- 5.Violin JD, Lefkowitz RJ. Beta-arrestin-biased ligands at seven-transmembrane receptors. Trends Pharmacol Sci. 2007;28:416–422. doi: 10.1016/j.tips.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 6.Rajagopal S, Ahn S, Rominger DH, Gowen-MacDonald W, Lam CM, DeWire SM, Violin JD, Lefkowitz RJ. Quantifying Ligand Bias at Seven-Transmembrane Receptors. Molecular Pharmacology. 2011;80:367–377. doi: 10.1124/mol.111.072801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rajagopal S, Rajagopal K, Lefkowitz RJ. Teaching old receptors new tricks: biasing seven-transmembrane receptors. Nat Rev Drug Discov. 2010;9:373–386. doi: 10.1038/nrd3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu JJ, Horst R, Katritch V, Stevens RC, Wuthrich K. Biased Signaling Pathways in 2-Adrenergic Receptor Characterized by 19F-NMR. Science. 2012;335:1106–1110. doi: 10.1126/science.1215802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saulière A, Bellot M, Paris H, Denis C, Finana F, Hansen JT, Altié MF, Seguelas MH, Pathak A, Hansen JL, Sénard JM, Galés C. Deciphering biased-agonism complexity reveals a new active AT1 receptor entity. Nat Chem Biol. 2012;8:622–630. doi: 10.1038/nchembio.961. [DOI] [PubMed] [Google Scholar]
- 10.Raehal KM, Walker JKL, Bohn LM. Morphine side effects in beta-arrestin 2 knockout mice. J Pharmacol Exp Ther. 2005;314:1195–1201. doi: 10.1124/jpet.105.087254. [DOI] [PubMed] [Google Scholar]
- 11.Palmer AE, Qin Y, Park JG, McCombs JE. Design and application of genetically encoded biosensors. Trends Biotechnol. 2011;29:144–152. doi: 10.1016/j.tibtech.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ibraheem A, Campbell RE. Designs and applications of fluorescent protein-based biosensors. Current Opinion in Chemical Biology. 2010;14:30–36. doi: 10.1016/j.cbpa.2009.09.033. [DOI] [PubMed] [Google Scholar]
- 13.akerboom J, Rivera JDV, Guilbe MMR, Malave ECA, Hernandez HH, Tian L, Hires SA, Marvin JS, looger LL, Schreiter ER. Crystal Structures of the GCaMP Calcium Sensor Reveal the Mechanism of Fluorescence Signal Change and Aid Rational Design. Journal of Biological Chemistry. 2008;284:6455–6464. doi: 10.1074/jbc.M807657200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Akerboom J, Chen TW, Wardill TJ, Tian L, Marvin JS, Mutlu S, Calderón NC, Esposti F, Borghuis BG, Sun XR, Gordus A, Orger MB, Portugues R, Engert F, Macklin JJ, Filosa A, Aggarwal A, Kerr RA, Takagi R, Kracun S, Shigetomi E, Khakh BS, Baier H, Lagnado L, Wang SSH, Bargmann CI, Kimmel BE, Jayaraman V, Svoboda K, Kim DS, Schreiter ER, Looger LL. Optimization of a GCaMP Calcium Indicator for Neural Activity Imaging. J Neurosci. 2012;32:13819–13840. doi: 10.1523/JNEUROSCI.2601-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakai J, Ohkura M, Imoto K. A high signal-to-noise Ca(2+) probe composed of a single green fluorescent protein. Nature Biotechnology. 2001;19:137–141. doi: 10.1038/84397. [DOI] [PubMed] [Google Scholar]
- 16.Zhao Y, Araki S, Wu J, Teramoto T, Chang YF, Nakano M, Abdelfattah AS, Fujiwara M, Ishihara T, Nagai T. An expanded palette of genetically encoded Ca2+ indicators. Science Signalling. 2011;333:1888. doi: 10.1126/science.1208592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barnett L, Platisa J, Popovic M, Pieribone VA, Hughes T. A fluorescent, genetically-encoded voltage probe capable of resolving action potentials. PLoS ONE. 2012;7:e43454. doi: 10.1371/journal.pone.0043454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nausch LWM, Ledoux J, Bonev AD, Nelson MT, Dostmann WR. Differential patterning of cGMP in vascular smooth muscle cells revealed by single GFP-linked biosensors. Proceedings of the National Academy of Sciences. 2007;105:365. doi: 10.1073/pnas.0710387105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tewson P, Westenberg M, Zhao Y, Campbell RE, Quinn AM, Hughes TE. Simultaneous Detection of Ca2+ and Diacylglycerol Signaling in Living Cells. PLoS ONE. 2012;7:e42791. doi: 10.1371/journal.pone.0042791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shaw G, Morse S, Ararat M, Graham FL. Preferential transformation of human neuronal cells by human adenoviruses and the origin of HEK 293 cells. The FASEB Journal. 2002;16:869–871. doi: 10.1096/fj.01-0995fje. [DOI] [PubMed] [Google Scholar]
- 21.Dries DR, Gallegos LL, Newton AC. A single residue in the C1 domain sensitizes novel protein kinase C isoforms to cellular diacylglycerol production. J Biol Chem. 2007;282:826–830. doi: 10.1074/jbc.C600268200. [DOI] [PubMed] [Google Scholar]
- 22.Giorgione JR, Lin JH, McCammon JA, Newton AC. Increased membrane affinity of the C1 domain of protein kinase Cdelta compensates for the lack of involvement of its C2 domain in membrane recruitment. J Biol Chem. 2006;281:1660–1669. doi: 10.1074/jbc.M510251200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jensen JB, Lyssand JS, Hague C, Hille B. Fluorescence changes reveal kinetic steps of muscarinic receptor-mediated modulation of phosphoinositides and Kv7.2/7.3 K+ channels. J Gen Physiol. 2009;133:347–359. doi: 10.1085/jgp.200810075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Falkenburger BH, Jensen JB, Hille B. Kinetics of M1 muscarinic receptor and G protein signaling to phospholipase C in living cells. J Gen Physiol. 2010;135:81–97. doi: 10.1085/jgp.200910344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alford SC, Abdelfattah AS, Ding Y, Campbell RE. A Fluorogenic Red Fluorescent Protein Heterodimer. Chem Biol. 2012;19:353–360. doi: 10.1016/j.chembiol.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kavran JM, Klein DE, Lee A, Falasca M, Isakoff SJ, Skolnik EY, Lemmon MA. Specificity and promiscuity in phosphoinositide binding by pleckstrin homology domains. J Biol Chem. 1998;273:30497–30508. doi: 10.1074/jbc.273.46.30497. [DOI] [PubMed] [Google Scholar]
- 27.van der Wal J. Monitoring Agonist-induced Phospholipase C Activation in Live Cells by Fluorescence Resonance Energy Transfer. Journal of Biological Chemistry. 2001;276:15337–15344. doi: 10.1074/jbc.M007194200. [DOI] [PubMed] [Google Scholar]
- 28.White PJ, Webb TE, Boarder MR. Characterization of a Ca2+ response to both UTP and ATP at human P2Y11 receptors: evidence for agonist-specific signaling. Molecular Pharmacology. 2003;63:1356–1363. doi: 10.1124/mol.63.6.1356. [DOI] [PubMed] [Google Scholar]
- 29.Zhang J, Chung T, Oldenburg K. A Simple Statistical Parameter for Use in Evaluation and Validation of High Throughput Screening Assays. Journal of Biomolecular Screening. 1999;4:67–73. doi: 10.1177/108705719900400206. [DOI] [PubMed] [Google Scholar]
- 30.Wu J, Liu L, Matsuda T, Zhao Y, Rebane A, Drobizhev M, Chang Y-F, Araki S, Arai Y, March K, Hughes T, Sagou K, Miyata T, Nagai T, Li W-H, Campbell RE. Improved orange and red Ca2+ indicators and photophysical considerations for optogenetic applications. ACS Chem Neurosci. 2013 doi: 10.1021/cn400012b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kost TA, Condreay JP, Ames RS, Rees S, Romanos MA. Implementation of BacMam virus gene delivery technology in a drug discovery setting. Drug Discov Today. 2007;12:396–403. doi: 10.1016/j.drudis.2007.02.017. [DOI] [PubMed] [Google Scholar]
- 32.Schroeder KS. FLIPR: A New Instrument for Accurate, High Throughput Optical Screening. Journal of Biomolecular Screening. 1996;1:75–80. [Google Scholar]
- 33.Poul EL, Hisada S, Mizuguchi Y, Dupriez VJ, Burgeon E, Detheux M. Adaptation of Aequorin Functional Assay to High Throughput Screening. Journal of Biomolecular Screening. 2002;7:57–65. doi: 10.1177/108705710200700108. [DOI] [PubMed] [Google Scholar]
- 34.Conklin BR, Farfel Z, Lustig KD, Julius D, Bourne HR. Substitution of three amino acids switches receptor specificity of Gq alpha to that of Gi alpha. Nature. 1993;363:274–276. doi: 10.1038/363274a0. [DOI] [PubMed] [Google Scholar]