Fig. 3.
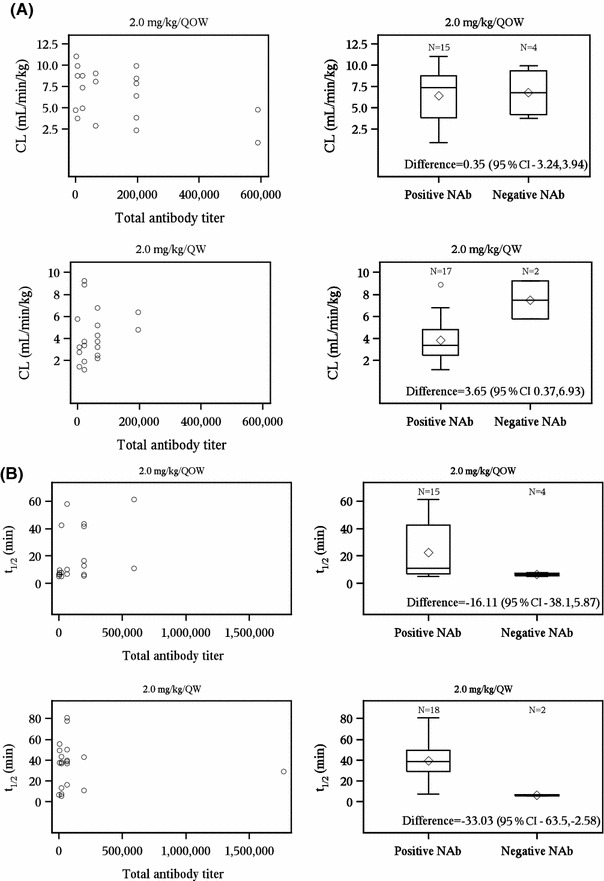
Association between elosulfase alfa pharmacokinetics and patient immunogenicity status. Pharmacokinetic values are from week 22 and patient immunogenicity status is from week 24. a Elosulfase alfa clearance and immunogenicity. b Elosulfase alfa plasma elimination half-life and immunogenicity. The bottom and top of whiskers represents the minimum and maximum values excluding outliers, which are plotted separately. The lower and upper ends of the box represent the 1st and 3rd quartile, the bar within the box represents the median value and the diamond within the box represents the mean value. CL total clearance of drug after intravenous administration, NAb neutralizing elosulfase alfa-specific antibodies that inhibit cellular receptor binding, TAb total anti-elosulfase alfa antibody, QOW every other week, QW every week
