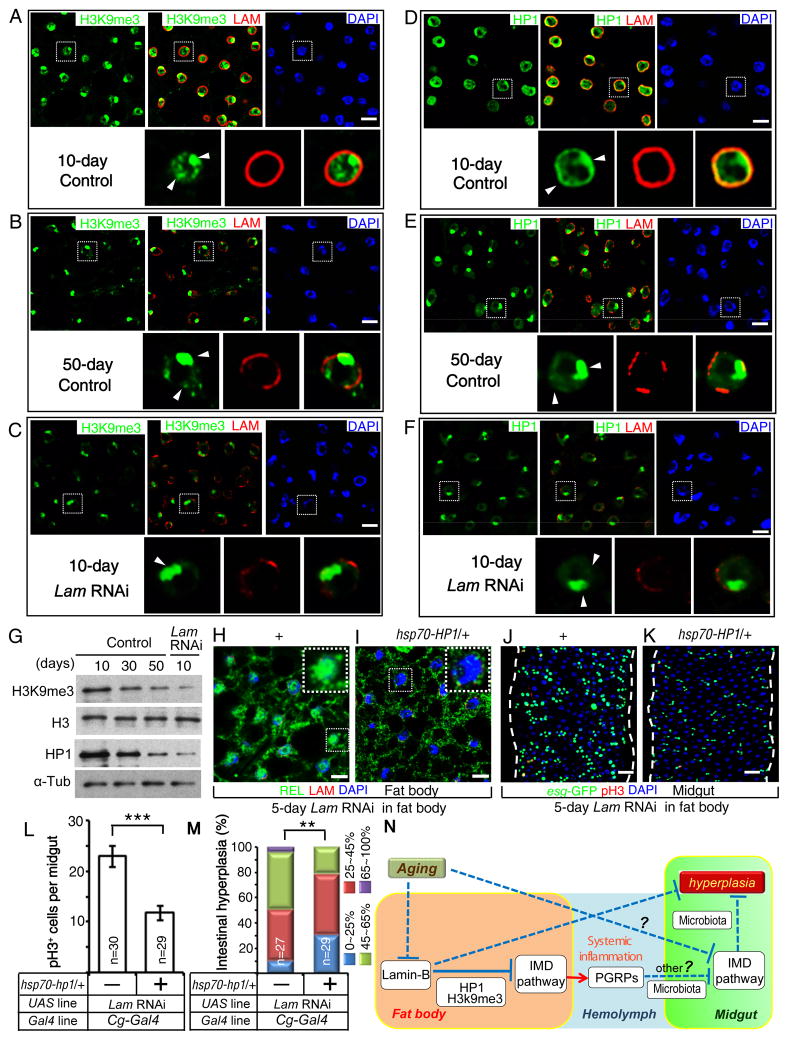
Figure 7. LAM represses systemic inflammation by maintaining fat body heterochromatin.
A–F. Compared to young wild-type fat bodies (A, D), reduction of H3K9me3 (B) and HP1 (E) in old wild-type fat bodies was mimicked by fat body specific LAM RNAi in young flies (C, F) under conventional condition. LAM, red; H3K9me3, green; HP1, green; DAPI, blue. Nuclei outlined by dashed boxes were enlarged at the bottom of each panel. Arrowheads, H3K9me3 or HP1 staining. Scale bars, 10 μm.
G. Western blotting of fat bodies revealed a reduction of H3K9me3 and HP1 upon aging or by fat-body specific LAM RNAi under conventional condition. Loading controls, Histone 3 (H3), α-tubulin (α-Tub).
H–I. Enhanced fat body IMD signaling (increased nuclear REL) in flies depleted of fat body LAM (H) was reversed by HP1 expression from the basal activity of Hsp70 promoter (I) under conventional condition. REL, green; LAM, red (missing due to RNAi); DAPI, blue. Nuclei boxed by dashed lines were enlarged to highlight the high (H) or low (I) nuclear REL. Scale bars, 10 μm.
J–K. Enhanced midgut hyperplasia in flies depleted of fat body LAM (J) was reversed by low level of HP1 expression (K). esg-GFP, green; pH3, red; DAPI, blue. Scale bars, 20 μm.
L. HP1-mediated reduction of midgut cell proliferation. The average number of pH3+ cells in the whole midgut was counted. n, number of midguts analyzed. Error bars, SEM. Student’s t-tests, ***p<0.001.
M. HP1-mediated reduction of midgut hyperplasia. The percent of esg-GFP+ cells (% of intestinal hyperplasia) was grouped into four classes and plotted. n, number of midguts analyzed. **p<0.01, Wilcoxon two-sample test.
N. A model. Aging associated lamin-B loss in fat body causes the loss of heterochromatin and de-repression of genes involved in fat body IMD signaling. This leads to increased fat body secretion of PGRPs and midgut IMD repression, which in turn causes gut hyperplasia. The effect of midgut IMD signaling by fat body PGRPs is mediated by microbiota dependent and independent pathways. Lamin-B loss in old fat body also causes midgut hyperplasia in microbiota and IMD dependent and independent pathways. Our studies do not rule out the possibility that aging fat body could cause midgut hyperplasia independent of lamin-B loss.
See also Figure S7.