Abstract
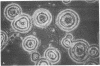
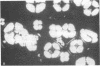
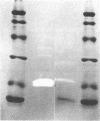
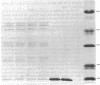
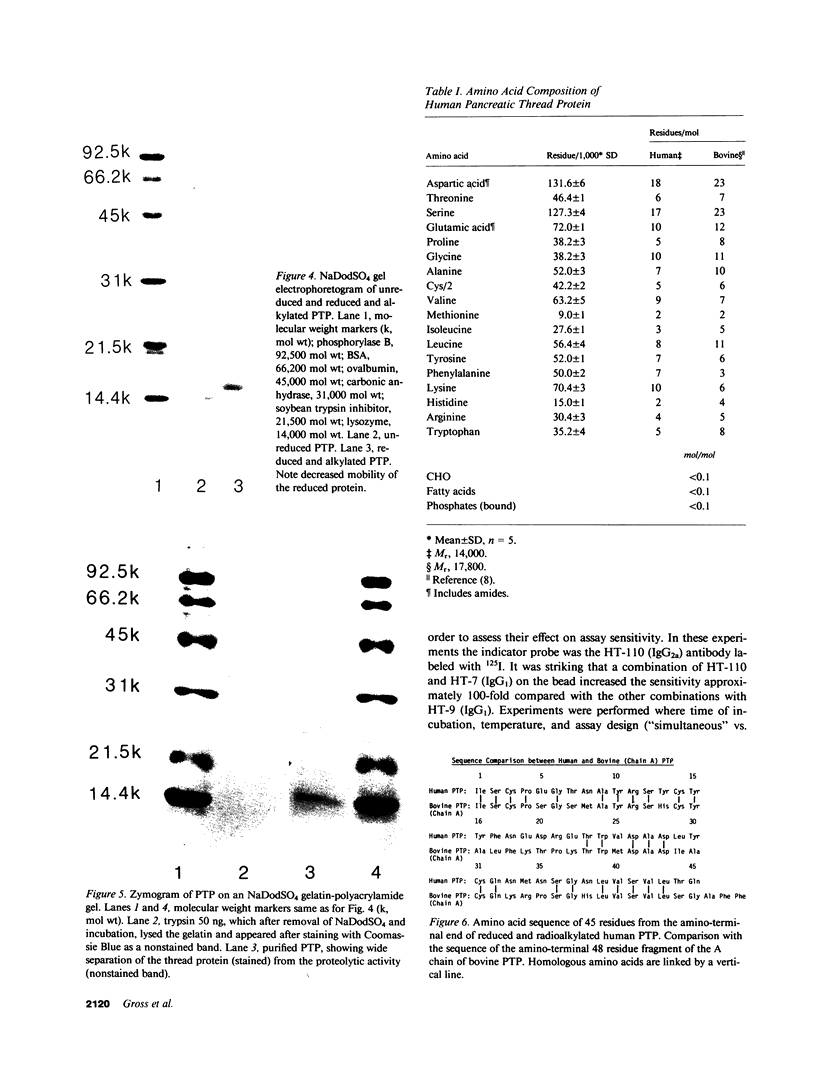
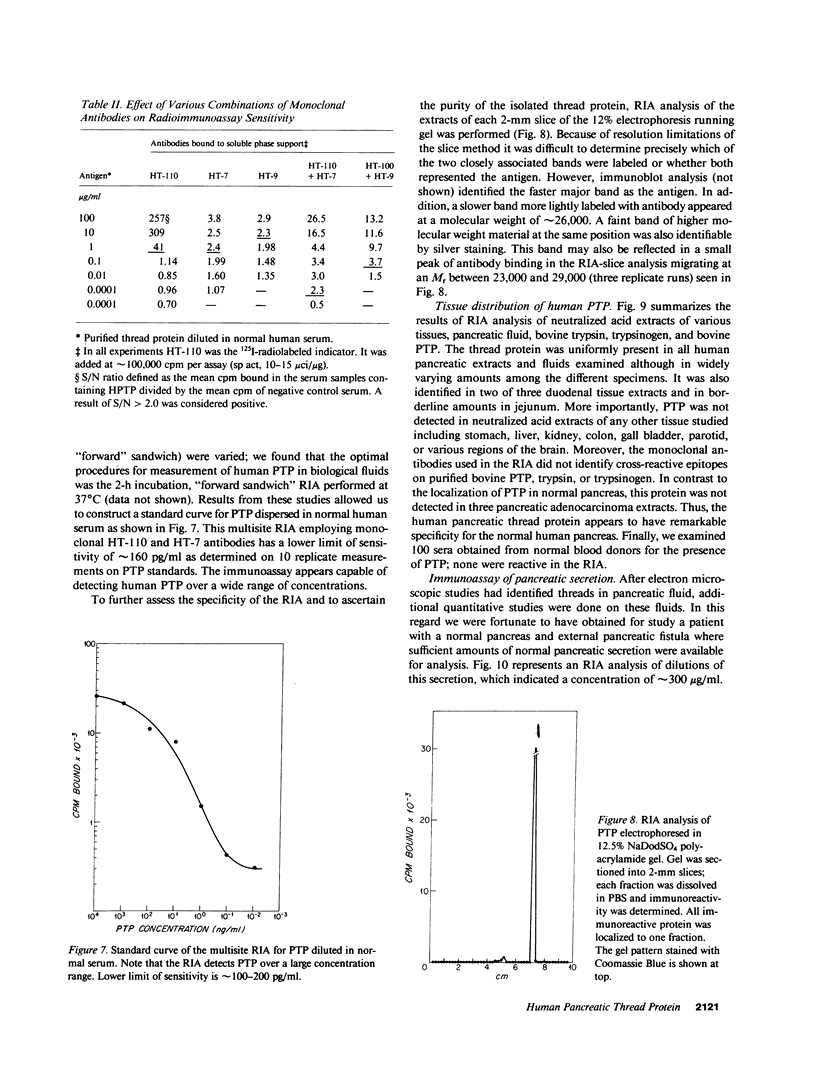
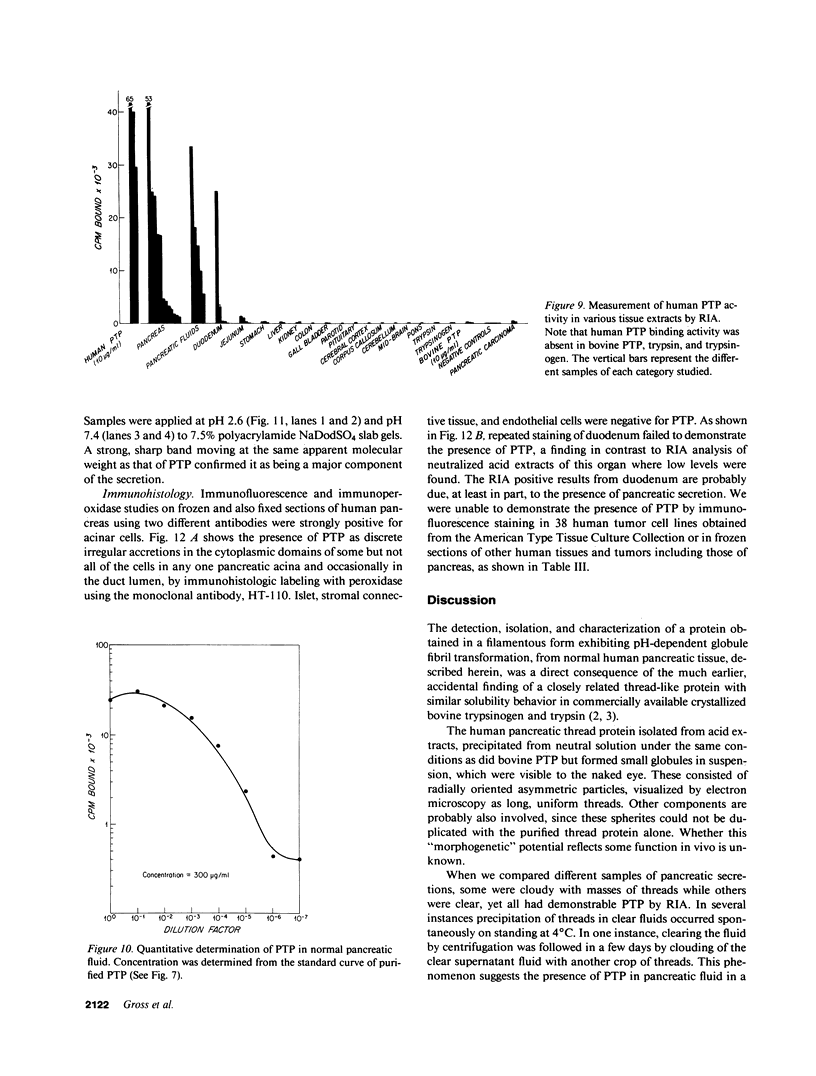
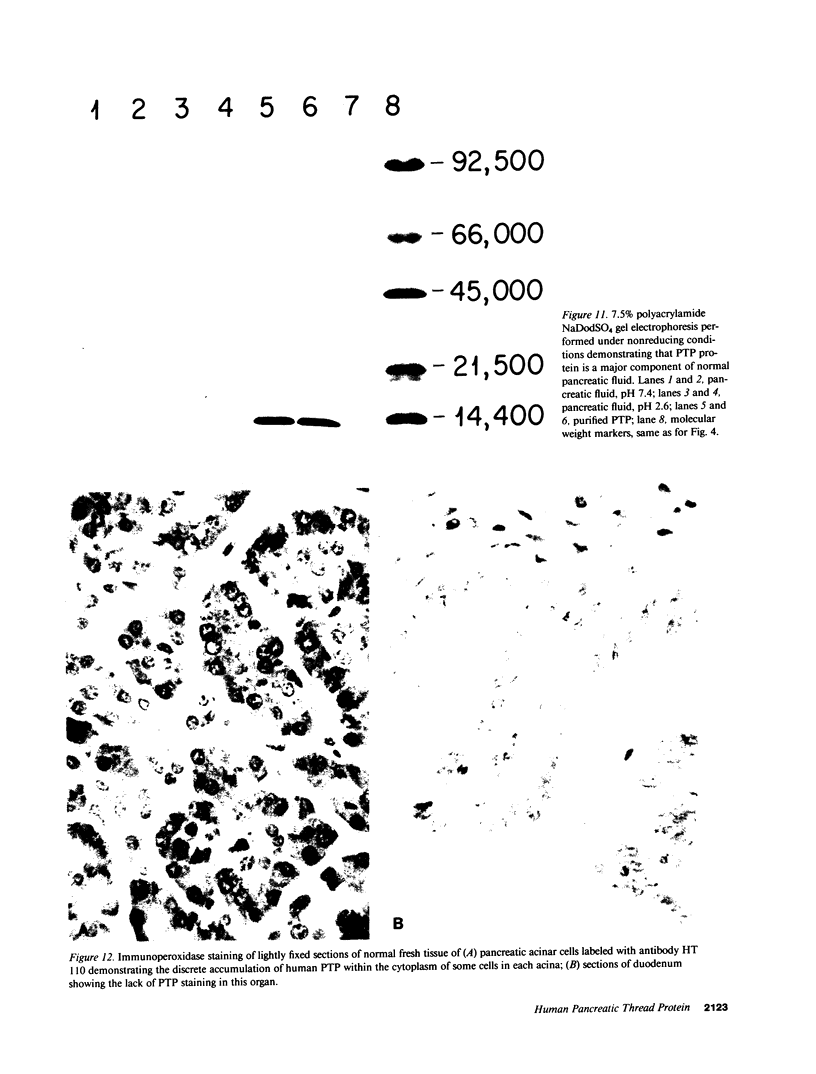
An unusual protein was isolated from acid extracts of normal human pancreas and pancreatic secretion in the form of uniform 7-10-nm long single threads without visible axial periodicity or other structure, as seen in the electron microscope. It accounts for as much as 300 micrograms/ml in some pancreatic secretions as measured by specific radioimmunoassay. The protein undergoes a freely reversible, pH dependent, globule-fibril transformation, being stable in the fibril form between pH 5.4 and 9.2. The monomer at acid pH has an apparent molecular weight of approximately 14,000 and consists of a single polypeptide chain, the amino acid composition of which is rich in aromatic amino acids and lacks carbohydrate, fatty acid, and phosphate. The amino acid sequence of 45 residues from the amino terminus shows no homology with any other reported protein sequences other than that of the A chain of the bovine pancreas thread protein (reported elsewhere). A sensitive radioimmunoassay employing monoclonal antibodies against human pancreatic thread protein failed to detect the antigen in a wide range of human tissues other than pancreas, nor was the antigen measurable in normal human sera. Immunohistochemistry utilizing these antibodies revealed the antigen as a component of the cytoplasm of some but not all the pancreatic acinar cells. A physiologic function has not yet been determined for this protein.
Full text
PDF


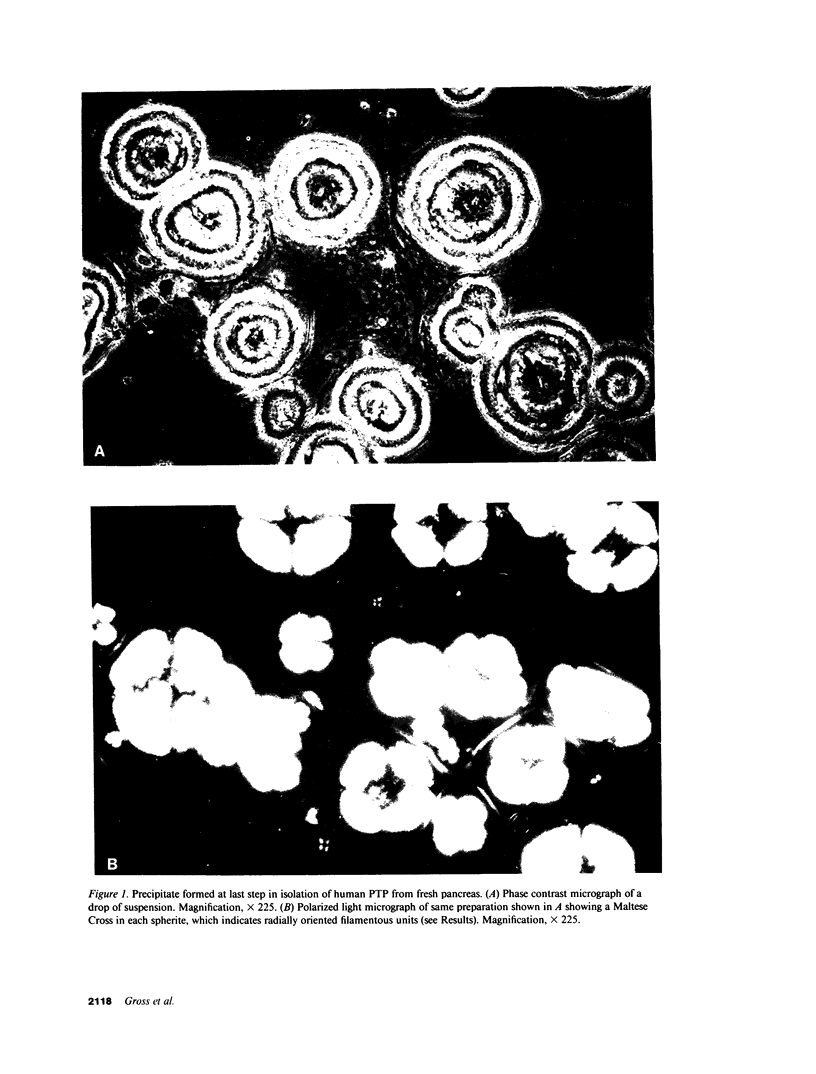
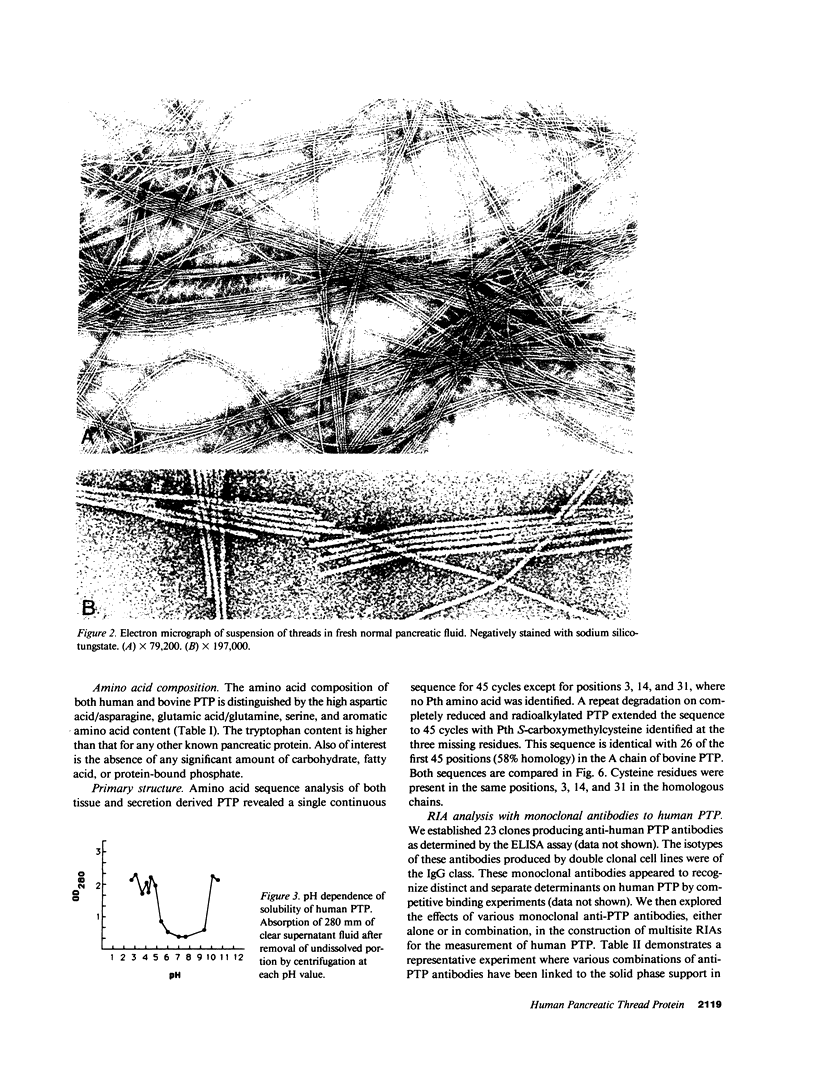



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailey K. Tropomyosin: a new asymmetric protein component of the muscle fibril. Biochem J. 1948;43(2):271–279. doi: 10.1042/bj0430271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauduin H., Stock C., Vincent D., Grenier J. F. Microfilamentous system and secretion of enzyme in the exocrine pancreas. Effect of cytochalasin B. J Cell Biol. 1975 Jul;66(1):165–181. doi: 10.1083/jcb.66.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellet D. H., Wands J. R., Isselbacher K. J., Bohuon C. Serum alpha-fetoprotein levels in human disease: perspective from a highly specific monoclonal radioimmunoassay. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3869–3873. doi: 10.1073/pnas.81.12.3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidart J. M., Ozturk M., Bellet D. H., Jolivet M., Gras-Masse H., Troalen F., Bohuon C. J., Wands J. R. Identification of epitopes associated with hCG and the beta hCG carboxyl terminus by monoclonal antibodies produced against a synthetic peptide. J Immunol. 1985 Jan;134(1):457–464. [PubMed] [Google Scholar]
- Carlson R. I., Ben-Porath E., Shouval D., Strauss W., Isselbacher K. J., Wands J. R. Antigenic characterization of human hepatocellular carcinoma. Development of in vitro and in vivo immunoassays that use monoclonal antibodies. J Clin Invest. 1985 Jul;76(1):40–51. doi: 10.1172/JCI111975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen-Solal L., Lian J. B., Kossiva D., Glimcher M. J. Identification of organic phosphorus covalently bound to collagen and non-collagenous proteins of chicken-bone matrix. The presence of O-phosphoserine and O-phosphothreonine in non-collagenous proteins, and their absence from phosporylated collagen. Biochem J. 1979 Jan 1;177(1):81–98. doi: 10.1042/bj1770081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Caro A., Lohse J., Sarles H. Characterization of a protein isolated from pancreatic calculi of men suffering from chronic calcifying pancreatitis. Biochem Biophys Res Commun. 1979 Apr 27;87(4):1176–1182. doi: 10.1016/s0006-291x(79)80031-5. [DOI] [PubMed] [Google Scholar]
- De Caro A., Multigner L., Lafont H., Lombardo D., Sarles H. The molecular characteristics of a human pancreatic acidic phosphoprotein that inhibits calcium carbonate crystal growth. Biochem J. 1984 Sep 15;222(3):669–677. doi: 10.1042/bj2220669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figarella C., Amouric M., Guy-Crotte O. Proteolysis of human trypsinogen 1. Pathogenic implication in chronic pancreatitis. Biochem Biophys Res Commun. 1984 Jan 13;118(1):154–161. doi: 10.1016/0006-291x(84)91080-5. [DOI] [PubMed] [Google Scholar]
- Fraker P. J., Speck J. C., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun. 1978 Feb 28;80(4):849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- GROSS J. Fiber formation in trypsinogen solutions; an electron optical study. Proc Soc Exp Biol Med. 1951 Oct;78(1):241–244. doi: 10.3181/00379727-78-19034. [DOI] [PubMed] [Google Scholar]
- Gross J., Brauer A. W., Bringhurst R. F., Corbett C., Margolies M. N. An unusual bovine pancreatic protein exhibiting pH-dependent globule-fibril transformation and unique amino acid sequence. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5627–5631. doi: 10.1073/pnas.82.17.5627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy-Crotte O., Amouric M., Figarella C. Characterization and N-terminal sequence of a degradation product of 14,000 molecular weight isolated from human pancreatic juice. Biochem Biophys Res Commun. 1984 Dec 14;125(2):516–523. doi: 10.1016/0006-291x(84)90570-9. [DOI] [PubMed] [Google Scholar]
- Guy O., Robles-Diaz G., Adrich Z., Sahel J., Sarles H. Protein content of precipitates present in pancreatic juice of alcoholic subjects and patients with chronic calcifying pancreatitis. Gastroenterology. 1983 Jan;84(1):102–107. [PubMed] [Google Scholar]
- Harris E. D., Jr, Krane S. M. An endopeptidase from rheumatoid synovial tissue culture. Biochim Biophys Acta. 1972 Feb 28;258(2):566–576. doi: 10.1016/0005-2744(72)90249-5. [DOI] [PubMed] [Google Scholar]
- He L., Isselbacher K. J., Wands J. R., Goodman H. M., Shih C., Quaroni A. Establishment and characterization of a new human hepatocellular carcinoma cell line. In Vitro. 1984 Jun;20(6):493–504. doi: 10.1007/BF02619623. [DOI] [PubMed] [Google Scholar]
- Heussen C., Dowdle E. B. Electrophoretic analysis of plasminogen activators in polyacrylamide gels containing sodium dodecyl sulfate and copolymerized substrates. Anal Biochem. 1980 Feb;102(1):196–202. doi: 10.1016/0003-2697(80)90338-3. [DOI] [PubMed] [Google Scholar]
- Hsu S. M., Raine L., Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981 Apr;29(4):577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lazarides E. Intermediate filaments as mechanical integrators of cellular space. Nature. 1980 Jan 17;283(5744):249–256. doi: 10.1038/283249a0. [DOI] [PubMed] [Google Scholar]
- Liu T. Y., Chang Y. H. Hydrolysis of proteins with p-toluenesulfonic acid. Determination of tryptophan. J Biol Chem. 1971 May 10;246(9):2842–2848. [PubMed] [Google Scholar]
- Merz P. A., Rohwer R. G., Kascsak R., Wisniewski H. M., Somerville R. A., Gibbs C. J., Jr, Gajdusek D. C. Infection-specific particle from the unconventional slow virus diseases. Science. 1984 Jul 27;225(4660):437–440. doi: 10.1126/science.6377496. [DOI] [PubMed] [Google Scholar]
- Merz P. A., Somerville R. A., Wisniewski H. M., Manuelidis L., Manuelidis E. E. Scrapie-associated fibrils in Creutzfeldt-Jakob disease. Nature. 1983 Dec 1;306(5942):474–476. doi: 10.1038/306474a0. [DOI] [PubMed] [Google Scholar]
- Mikes O., Holeysovský V., Tomásek V., Sorm F. Covalent structure of bovine trypsinogen. The position of the remaining amides. Biochem Biophys Res Commun. 1966 Aug 12;24(3):346–352. doi: 10.1016/0006-291x(66)90162-8. [DOI] [PubMed] [Google Scholar]
- Olsen B. R., Svenneby G., Kvamme E., Tveit B., Eskeland T. Formation and ultrastructure of enzymically active polymers of pig renal glutaminase. J Mol Biol. 1970 Sep 14;52(2):239–245. doi: 10.1016/0022-2836(70)90028-8. [DOI] [PubMed] [Google Scholar]
- Prusiner S. B., McKinley M. P., Bowman K. A., Bolton D. C., Bendheim P. E., Groth D. F., Glenner G. G. Scrapie prions aggregate to form amyloid-like birefringent rods. Cell. 1983 Dec;35(2 Pt 1):349–358. doi: 10.1016/0092-8674(83)90168-x. [DOI] [PubMed] [Google Scholar]
- Scheele G., Bartelt D., Bieger W. Characterization of human exocrine pancreatic proteins by two-dimensional isoelectric focusing/sodium dodecyl sulfate gel electrophoresis. Gastroenterology. 1981 Mar;80(3):461–473. [PubMed] [Google Scholar]
- Selkoe D. J., Ihara Y., Salazar F. J. Alzheimer's disease: insolubility of partially purified paired helical filaments in sodium dodecyl sulfate and urea. Science. 1982 Mar 5;215(4537):1243–1245. doi: 10.1126/science.6120571. [DOI] [PubMed] [Google Scholar]
- Smith J. A., Margolies M. N. Complete amino acid sequence of the heavy-chain variable region from an A/J mouse antigen-nonbinding monoclonal antibody bearing the predominant arsonate idiotype. Biochemistry. 1984 Sep 25;23(20):4726–4732. doi: 10.1021/bi00315a031. [DOI] [PubMed] [Google Scholar]
- Trelstad R. L., Lawley K. R. A simple economical buffer system for amino acid analysis. Anal Biochem. 1976 Jan;70(1):287–289. doi: 10.1016/s0003-2697(76)80073-5. [DOI] [PubMed] [Google Scholar]
- Wands J. R., Bruns R. R., Carlson R. I., Ware A., Menitove J. E., Isselbacher K. J. Monoclonal IgM radioimmunoassay for hepatitis B surface antigen: high binding activity in serum that is unreactive with conventional antibodies. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1277–1281. doi: 10.1073/pnas.79.4.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wands J. R., Marciniak R. A., Isselbacher K. J., Varghese M., Don G., Halliday J. W., Powell L. W. Demonstration of previously undetected hepatitis B viral determinants in an Australian Aboriginal population by monoclonal anti-hbs antibody radioimmunoassays. Lancet. 1982 May 1;1(8279):977–980. doi: 10.1016/s0140-6736(82)91988-2. [DOI] [PubMed] [Google Scholar]
- Wands J. R., Wong M. A., Shorey J., Brown R. D., Marciniak R. A., Isselbacher K. J. Hepatitis B viral antigenic structure: signature analysis by monoclonal radioimmunoassays. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2237–2241. doi: 10.1073/pnas.81.7.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wands J. R., Zurawski V. R., Jr High affinity monoclonal antibodies to hepatitis B surface antigen (HBsAg) produced by somatic cell hybrids. Gastroenterology. 1981 Feb;80(2):225–232. [PubMed] [Google Scholar]
- de Haas G. H., Slotboom A. J., Bonsen P. P., van Deenen L. L. Studies on phospholipase A and its zymogen from porcine pancreas. I. The complete amino acid sequence. Biochim Biophys Acta. 1970 Oct 20;221(1):31–53. doi: 10.1016/0005-2795(70)90195-9. [DOI] [PubMed] [Google Scholar]