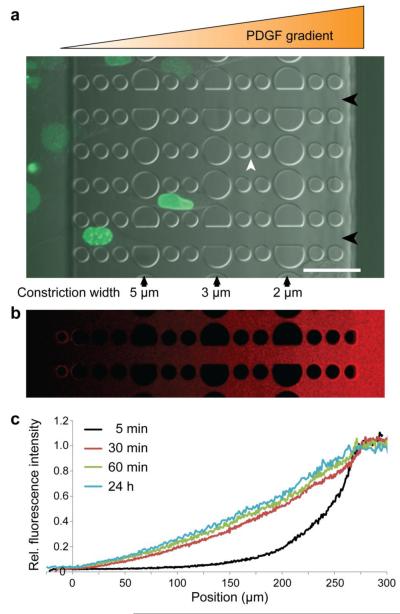
Figure 1. Overview of the microfluidic migration device.
a) Cells are seeded in a 250 μm tall chamber of the device (far left), which is separated from the other chamber (far right) by 5 μm tall constriction channels consisting of PDMS pillars. Larger pillars create a series of 5 μm, 3 μm, and 2 μm wide constrictions (black arrows). In the direction perpendicular to the gradient, 2 wide spacings between pillars (white arrowhead) allow the gradient to form uniformly even when cells are inside the constriction channels. Channels with wider spacing (15 μm, black arrowheads) are provided to assess the cell migration speed without nuclear confinement. Shown here are human skin fibroblasts expressing GFP-lamin A migrating through a device towards a PDGF chemotactic gradient. Scale bar: 50 μm. b) Gradient formation after 24 hours of fluorescently labeled (Texas Red) 70 kD dextran. c) Fluorescence intensity across the migration device during gradient formation with 70 kD fluorescent dextran, demonstrating that the gradient forms quickly (within 30 minutes) and is stable over 24 hours.