Figure 2.
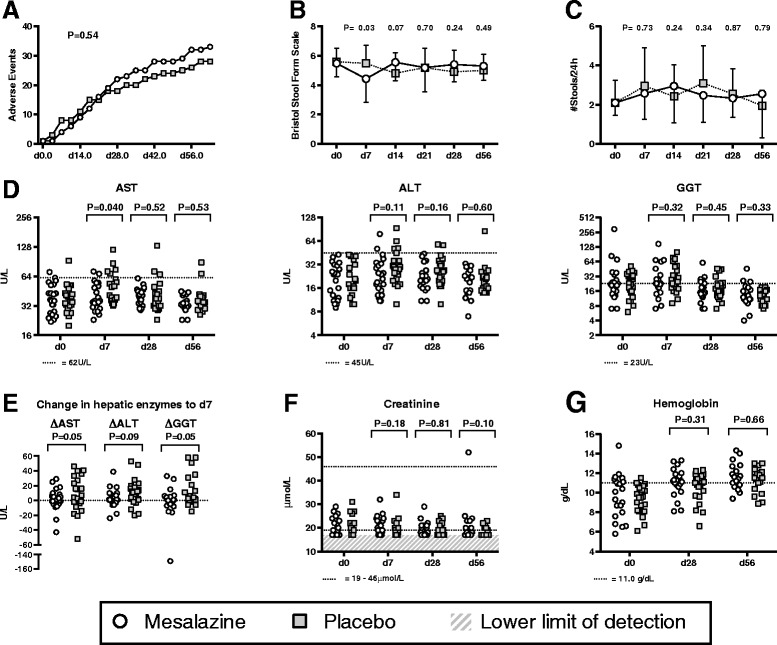
Safety and toxicity. Cumulative timing of adverse events between the arms (A). Stool consistency (Bristol Stool Form Scale) and frequency in the 24 hours preceding clinical review (B,C). Hepatic enzymes, aspartate aminotransferase (AST), alanine transaminase (ALT) and gamma-glutamyl transpeptidase (GGT), for all participants in the study (D). Change in hepatic enzymes from baseline to day 7 (E). Creatinine and hemoglobin for all participants (F,G). Differences between arms at baseline are highlighted if P <0.1, upper limit of normal (alongside lower limit for creatinine) illustrated where appropriate.
