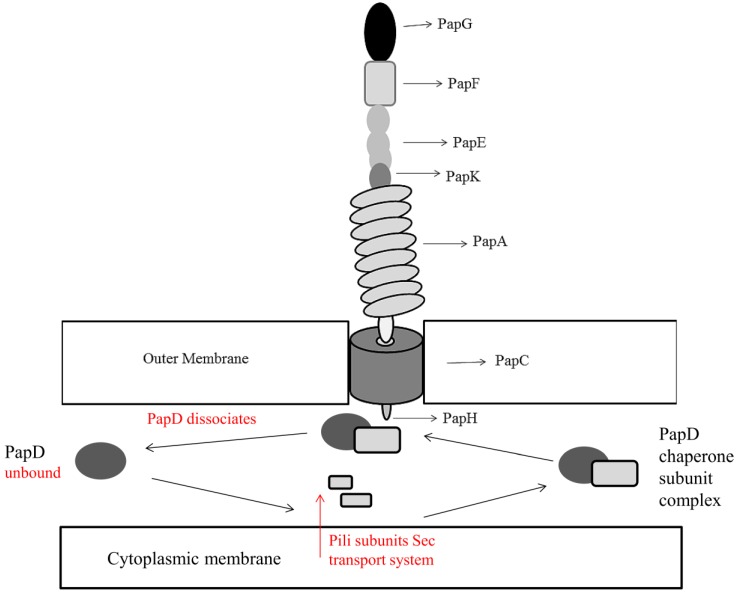
Figure 2.
Structure of Escherichia coli Type P pili encompassing the PapG unit containing galabiose specific receptors (Gal(α1–4)Gal-) for attachment to urinary tract tissue. The pilus is anchored to the membrane by PapH, whose location is yet to be characterized fully but has been hypothesized by Verger and colleagues to terminate the pilus structure at the base as shown, allowing anchoring to the membrane [46]. Type P pili subunits enter the periplasm by the Sec transport system. In the presence of PapD, stable chaperone-subunit complexes are formed via attachment to the hydrophobic C-terminus of pili subunits [47]. PapD acts as the chaperone to assemble and deliver pili subunits to the outer membrane usher PapC. PapC is a pore forming protein that facilitates pilus assembly by creating a narrow channel across the outer membrane. Assembly of subunits from the outer membrane PapC occurs through a donor strand exchange mechanism. PapA forms a tightly wound helix fiber on the external cell and provides a driving force for the translocation of pili subunits across the outer membrane, facilitating outward pilus growth [48]. Adapted from Mu and Bullitt, 2006 [48] and Mu, 2005 [49].