Abstract
Tetratheca juncea Smith (Tremandraceae) has undergone a range contraction of approx. 50 km in the last 100 years and is now listed as a vulnerable sub‐shrub restricted to the central and north coast regions of New South Wales, Australia. There are approx. 250 populations in a 110 km north–south distribution and populations are usually small with fewer than 50 plants/clumps. The reproductive ecology of the species was studied to determine why seed‐set is reportedly rare. Flowers are bisexual, odourless and nectarless. Flowers are presented dependentally and there are eight stamens recurved around the pistil. Anthers are poricidal, contain viable pollen and basally contain a deep‐red tapetal fluid that is slightly oily. Thus flowers are presented for buzz pollinators, although none were observed at flowers during our study. The species was found to be facultatively xenogamous with only one in 50 glasshouse flowers setting seed autogamously, i.e. without pollinator assistance. Field studies revealed fertile fruit in 24 populations but production varied significantly across sites from exceedingly low (0·6 fruits per plant clump) to low (17 fruits per plant clump). Fruit‐set ranged from 0 to 65 %, suggesting that pollen vectors exist or that autogamy levels in the field are variable and higher than glasshouse results. Fruit production did not vary with population size, although in three of the five populations in the south‐west region more than twice as much fruit was produced as in populations elsewhere. A moderately strong relationship between foliage volume and fruit : flower ratios suggests that bigger plants may be more attractive than smaller plants to pollinators. A review of Tetratheca pollination ecology revealed that several species are poorly fecund and pollinators are rare. The habitat requirements for Tetratheca, a genus of many rare and threatened species, is discussed.
Key words: Poricidal anthers, reproductive success, population size, range decline, plant size, threatened species, Tetratheca juncea, Tremandraceae
INTRODUCTION
The genera Platytheca, Tetratheca and Tremandra comprise the small endemic Australian plant family Tremandraceae. Platytheca (three species) and Tremandra (two species) are found in south‐west Australia, while Tetratheca (39 species) is widely distributed across the southern region of the continent with just over half of the species occurring in Western Australia and the remainder in South Australia or eastern Australia (Thompson, 1976). Many of the species in Tetratheca are rare or threatened (32 % of the genus; Walter and Gillett, 1998), and two species are presumed extinct (Briggs and Leigh, 1996).
Tetratheca juncea is found only on the central and northern coasts of New South Wales (NSW). It was collected at Port Jackson and suburbs of Sydney during the late 1800s (Thompson, 1976) and 160 km north at Mt Bulladelah (Bulahdelah) (Thompson, 1976). The southern populations are now extinct and the species has contracted to a north–south range of approx. 110 km between north Wyong and Bulahdelah (Payne, 2000). Habitat clearing for housing is the primary cause of range contraction. The species is listed as vulnerable by The World Conservation Union (IUCN) (Walter and Gillett, 1998) and is considered vulnerable in the NSW Threatened Species Conservation Act (Schedule 2, TSC Act 1995), and in the Commonwealth Environment Protection and Biodiversity Conservation Act (transferred schedules from ESP Act 1992) and in Briggs and Leigh (1996).
Tetrathecajuncea is distinguished from other eastern Australian species by the distinctly angular, winged stem (Thompson, 1976). It is a straggling, usually leafless shrub and, in common with many other Tetratheca species, it has hanging purple flowers, with the dark centre giving rise to the common name ‘Black Eyed Susan’. Juvenile plants and regrowth stems usually have obovate leaves, but these are soon deciduous and rarely appear on older stems. An individual plant can grow into a clump of many stems (one to approx. 500 stems) of genets and ramets (Bartier et al., 2001). Clumps appear to be long‐lived with the inside of the clump becoming senescent while the outside of the clump remains vigorous. The proportion of genets to ramets in a clump and at the population level is unknown.
The species is found in open woodland and heath communities and generally overlaps with coal mining leases or land scheduled for urban development. The populations are usually small with less than 50 plants/clumps. Taxonomic information is available on the genus (Thompson, 1976, 1978; Boesewinkel 1999), but the ecology of the species is poorly understood. Management of this species depends on a sound knowledge of the species’ reproductive ecology, particularly given Payne’s report of nil to low levels of seed in many populations (Payne, 1998, p. 38). With this poor prognosis for T. juncea, the objective of our study was to determine if the low production of fruit is widespread and which factors (if any) influence fecundity in the species. Thus the breeding system, floral visitation and fecundity levels were examined in detail.
MATERIALS AND METHODS
Site and plant selection
Tetratheca juncea is known from 250 locations between north Wyong and Bulahdelah, NSW, Australia (Payne, cited in Bartier et al., 2001). The area in which the species is found was divided into four quadrants (NW, NE, SW and SE) and, to sample any geographic variation in traits, 25 populations were selected uniformly across these quadrants. One population was burnt by persons unknown during the study and was then excluded from most of the field studies. In addition, whole plant clumps were salvaged for study and transferred to glasshouses when, on occasions, populations were about to be destroyed by industry under an approved development consent. Plant clumps became the base unit for investigations.
Floral morphology
Whole plants from the SW and NW quadrant were grown in the glasshouse from September to December 2000 and flowers of T. juncea were collected, dissected and illustrated in order to describe the basic form of flowers and flowering patterns on stems. The types of rewards on offer for floral visitors, the mechanism of pollen dispersal and viability of pollen were assessed. Stigma receptivity was not examined because of the very small size of this structure. Pollen viability was tested on three flowers from each of three plants growing in the glasshouse using a 0·5 % solution of 2,3,5‐triphenyl tetrazolium chloride (TCC) in 12 % sucrose, after the method of Cook and Stanley (1960). Slides were left for 3 d to allow thorough infiltration of TCC into the pollen exine. Under 200× magnification, the first 200 grains viewed on a microscope slide were assessed as viable or inviable. Pollen stains red in the presence of reductases, indicating enzyme activity, and therefore red grains were counted as viable. Pollen killed in FPA (90 : 5 : 5 formalin : propionic acid : acetic acid) and soaked in the TCC solution provided a standard against which inviable pollen could be compared. Inviable pollen does not take up the stain.
Breeding system
To determine whether or not Tetratheca juncea obligately requires pollen vectors to effect seed‐set, the breeding system of salvaged plants from the NW (n = 7 plants, Newstan Colliery) and SW (n = 5 plants, Vales Point) populations were studied in a glasshouse. Three treatments were made on flowering plants: (1) bagging of mature buds to test for automatic self‐pollination (autogamy); (2) hand‐pollination of virgin flowers using self pollen (to test for self compatibility); (3) hand‐pollination of virgin flowers using pollen from an individual that in the field was growing at least 5 m away (to test for outcrossing ability). The hand‐pollination techniques used in Gross (1990) with modifications for poricidal anthers (Gross, 1993) were used. Flowers received only one application of pollen. A micro‐dissecting head lens and a dissecting microscope were used to check that pollen was transferred to stigmas. Pollen harvesting was made more difficult by the presence of tapetal fluid in anthers (see below). Fertile fruit were scored 3–4 weeks after treatment.
Reproductive success, plant size and population size
Twenty‐four populations, with at least five plants/clumps from each quadrant, were selected for field assays of flower and fruit‐production. Population size was investigated as a potential parameter influencing flower production and fruiting success. Thus very small (less than 15 plants/clumps, four sites), small (16–30 plants/clumps, eight sites), medium (31–60 plants/clumps, seven sites) and large (100+ plants/clumps, five sites) populations were incorporated into the study. Fruit‐set data were collected during one census between late spring 2000 and the summer of 2000–2001 for each of ten individual plant clumps in 22 sites and for all individual plant clumps in two populations with less than 10 clumps. At each plant clump, all buds, flowers and fruits were counted and an estimation made of the volume of plant foliage [π (circumference/2 π)2 × height]. For plants at the end of their flowering period (i.e. no buds or flowers remaining) fruit : flower ratios were calculated as a measure of an individual plant’s fertilization success. Only plants without buds or flowers were used and fruit : flower ratios per plant were calculated as total [fruit/mean (buds + flowers + fruits)]. In addition, at four populations, one from each quadrant, the mean number of seed per fruit (n = 8–18) was calculated for one to five plants/clumps.
Floral visitors
Observations of flowers to detect floral visitors were undertaken in 2000 on sunny days (approx. 1000 h to 1600 h) at most of the 25 study sites used for fruit assays (see above) and, in particular, at Glenrock Nature Reserve and Green Point Recreation Reserve. At the latter sites shrubs with more than 20 flowers were watched for set periods (15–25 min) and floral visitors were observed for identity, time spent at flower, activity at flower (buzzing anthers or not), the number of flowers visited per bush and the next species visited. Nearly 100 h of observation at the two reserves and a further 12 h of cursory observations were spent in the field watching flowers for floral visitors.
Statistical analyses
Reproductive data collected in populations were analysed with parametric fixed factor one‐way ANOVA and linear regression. Transformations (1/SQRT + 1·5) were required to make variances homoscedastic. Tukey’s HSD (honestly significantly different) test for unplanned pairwise comparisons was used as a post hoc test for fruit production. Data were analysed with Statgraphics Plus 2.0™.
RESULTS
Floral morphology
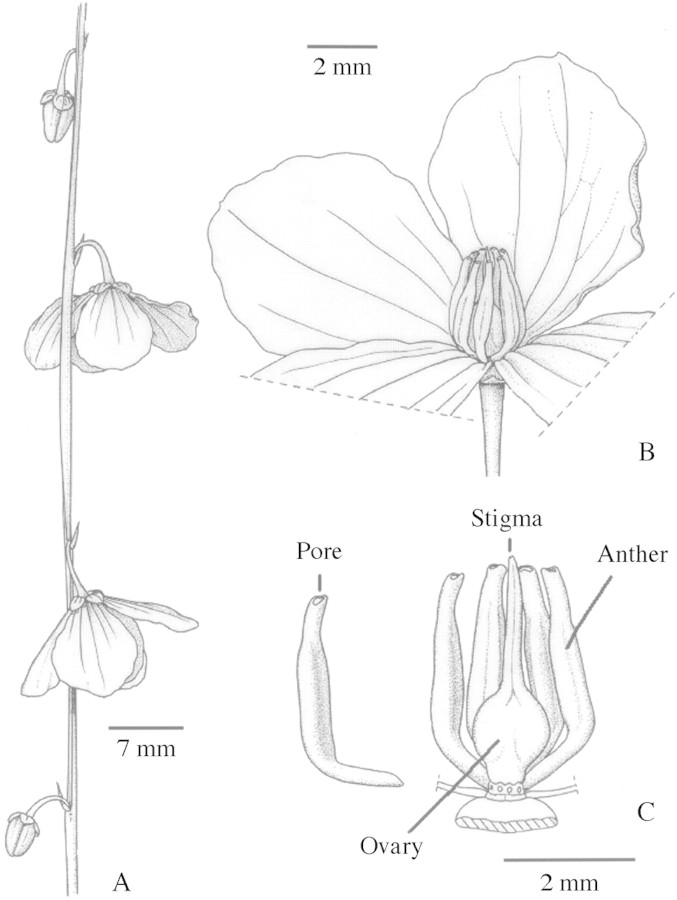
Most flowers of T. juncea (Fig. 1) have four petals and four ovules (five of each occasionally occur). Flowers are dependentally presented (hanging down), solitary or in pairs and alternately positioned along stems (Fig. 1). Flowering is indeterminate. The second flower in a pair often develops later such that a stem can have buds below and above open flowers (Fig. 1). Unfertilized flowers remain persistent on stems for several weeks. Flowers are bisexual, odourless and nectarless. There are eight anthers recurved around the pistil (Fig. 1). Anthers are poricidal and basally contain a deep‐red tapetal fluid that is slightly oily. The style projects from the corona of anthers and elongates with age. The stigma is minute (less than 0·2 mm wide). Pollen is 80 % viable at first but by 14 d is inviable. Only a few pollen grains are passively shed from the anthers (sometimes landing on the stigma), otherwise pollen has to be actively removed from anthers. The presence of poricidal anthers denotes that pollination involves bees capable of anther sonication (Buchmann, 1983). The presence of tapetal fluid further supports this hypothesis, as in other poricidal systems the gradual dehydration of tapetal fluid assists with a timed release of pollen to sonicating bees (King and Buchmann, 1996).
Fig. 1.Tetratheca juncea (A) floral stalk, (B) flower and (C) floral form.
Breeding system
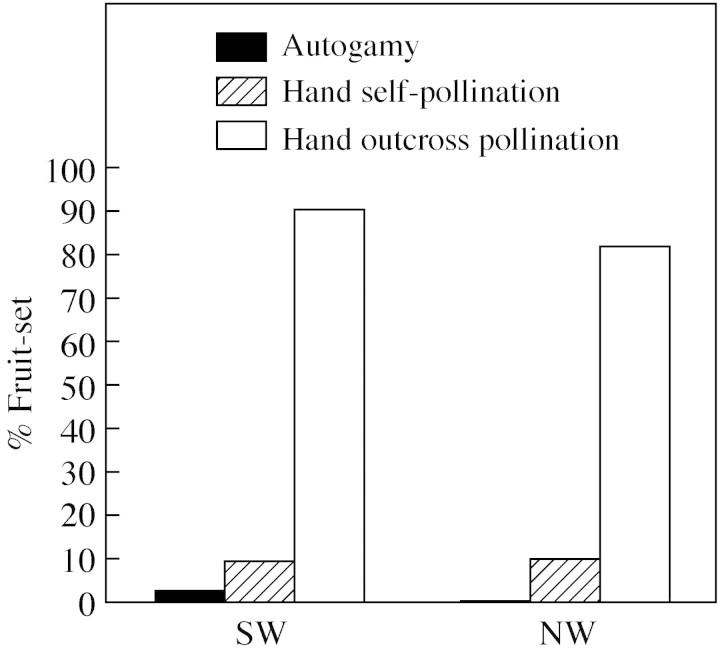
Tetratheca juncea’s predominate breeding system in both populations is a xenogamous one (Fig. 2) as most fruits were produced when pollen from a different individual was used in the hand‐pollination trials. However, seed are occasionally produced autogamously and about one flower in 50 will do this under glasshouse conditions.
Fig. 2. Breeding system results. Fruit‐set from plants from two quadrants using three pollination treatments in a glasshouse: automatic self‐pollination where flowers are tagged but not manipulated, self‐hand pollination where flowers are pollinated with their own pollen and outcross pollination where flowers are hand pollinated with pollen from a different plant.
Reproductive success, plant size and population size
Buds, flowers and fertile fruit were recorded in all of the 24 study populations. At the census time the total reproductive output (buds + flowers + fruit) varied significantly among populations (F23,226 = 4·61, P = 0·00001) and was found to have a significant negative relationship with population size (r2 = 24·09 %, P = 0·01).
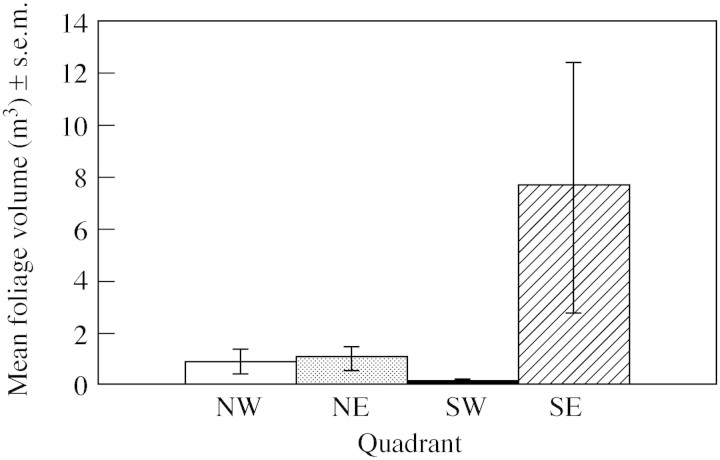
Total reproductive output was weakly associated with foliage volume (r2 = 16·42 %, P = 0·05), although across populations there was a significant trend for larger plants to bear more fruit than smaller ones (Fig. 3) with the two outlying populations coming from the SE quadrant. Foliage volume did vary significantly among quadrants (F3,20 = 6·61, P = 0·003) and there was a clear trend for plants in the SE to be much larger than elsewhere (Fig. 4).
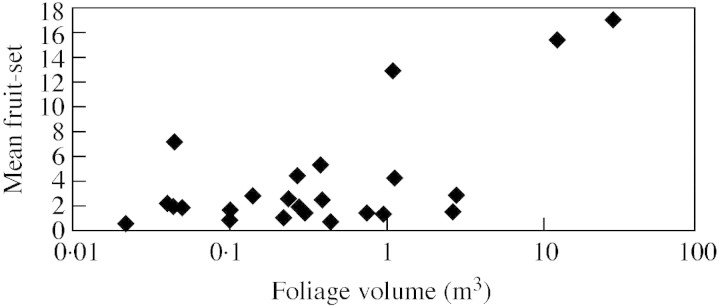
Fig. 3. A significant positive relationship was found with fruit production (mean fruit‐set) and plant biomass (foliage volume) where y = 5·69 + 1·571x LOG (foliage volume), r2 = 41·55 % and P = 0·0007.
Fig. 4. Variation in foliage volume (cm3) across quadrants. Kruskal–Wallis analyses showed that foliage volume did not vary significantly across sites (H = 1·50, P = 0·68).
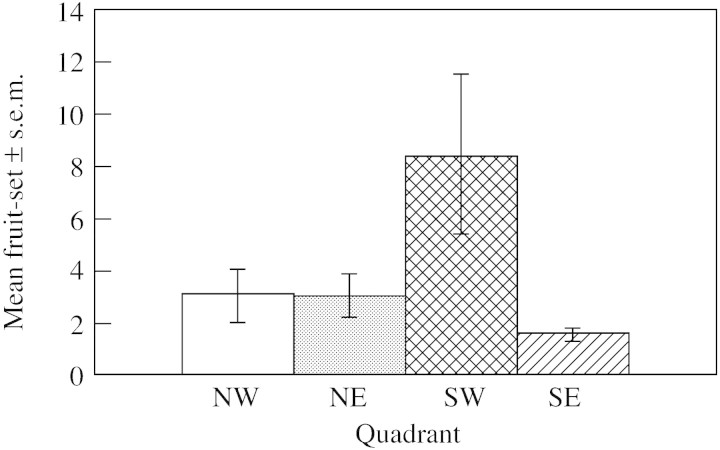
Fruit production also varied significantly across populations (F23,226 = 3·05, P = 0·00001) and among the 24 populations mean fruit production varied from 0·6 ± 0·4 fruits per clump to 17 ± 4·93 fruits per clump, although post hoc tests revealed that only one population was significantly different from most other sites. This population and two others from the five populations in the SW quadrant (Fig. 5) produced more than twice as much fruit as any other population (and Fig. 4 shows that this is not because of large plants).
Fig. 5. Mean fruit‐set ± s.e.m. across the four quadrants, NW (six populations), NE (five populations), SW (six populations) and SE (seven populations).
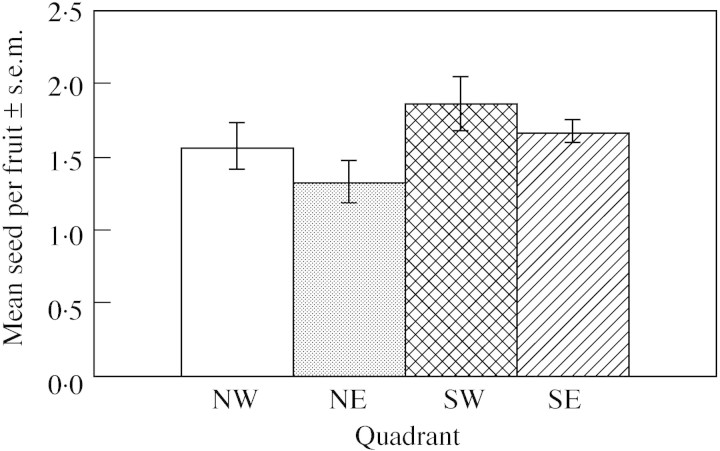
Seed production per fruit was also investigated in one population per quadrant and no statistical difference was found among quadrants (Fig. 6; H = 4·55, P = 0·21). These data were pooled and mean seed production per fruit (n = 45) across quadrants was 1·65 ± 0·91 seed per fruit. 1/SQRT fruit production was regressed against the log of population size but no statistically significant relationship was detected (r2 = 5·89 %, P = 0·25) even when fruit production was scaled for foliage volume (r2 = 0·01 %, P = 0·96).
Fig. 6. Mean seed ± s.e.m. produced in fruit in four populations one in each of the four quadrants (NW, NE, SW and SE).
Fruit : flower ratios were investigated for 32 plants (nine populations) where flowering and bud production had ceased. Fruit : flower ratios ranged from 0 to 0·65 and while there was no significant relationship between population size and fruit : flower ratios (r2 = 1·67 %, P = 0·81), a moderately strong relationship between foliage volume and fruit: flower ratio was detected (r2 = 77·10 %, P = 0·02) on fertile plants. Most populations with high fruit : flower ratios were in the SE quadrant.
Floral visitors
No bees were detected at flowers of T. juncea during our study. Lycaenid butterflies occasionally visited flowers but then did not touch the stigma while foraging. Native bees were not common at any site but they were seen regularly visiting other species at the sites and on adjacent plants to those being observed, indicating that our presence did not deter these bees from foraging.
DISCUSSION
Fertile fruits were recorded in all 24 populations. Fruit can be set automatically by the species but breeding experiments in the glasshouse showed that only 2 % of flowers will do this successfully. These low levels of autogamy come about because pollen is only rarely and passively dropped onto stigmas. For fruit‐set to reach higher levels, pollen has to be actively transferred from anthers to stigmas. This is because the pollen in T. juncea is secured inside poricidal anthers that do not ‘un‐zip’ and release their pollen, unlike most anthers in flowering plants. It has been well documented that plants with poricidal anthers are pollinated by bees capable of sonicating anthers (e.g. see Buchmann, 1983). These bees are called buzz‐pollinators. In Australia there are many species of native bee capable of buzz pollination (Gross and Mackay, 1998) and there are many plant species that require buzz pollination (e.g. species in Hibbertia, Dianella, Senna, Melastoma and Solanum).
In the study populations, fruit‐set ranged from 0 to 65 %, which strongly suggests that vector‐mediated pollination events are occurring in some populations. However, no bees were detected at flowers of T. juncea during this study, despite over 100 h of observations and at times there being up to five observers at sites where native bees were observed on other species. An occasional lycaenid butterfly was observed at flowers but then pollen transfer did not occur. [The ‘carpenter bee’ caught on T. juncea, referred to in Payne (2000, p. 7), was examined and identified by Gross to be a fly.] Across these 24 study sites, local decline of pollinators seems likely for T. juncea; although in other populations pollinators may be present. Broadening the survey to include additional sites may be beneficial and, as Williams et al. (2001) point out, intense sampling among sites and years may be required. For example, Steiner (1993) conceded the local loss of pollinators for Ixianthes retziodes E.Meyer ex Benth. (Scrophulariaceae) after 32 h of observation at different times of the day over 4 years and at five sites. Subsequently, the predicted pollinator was found in a recently discovered population of I. retziodes (Steiner and Whitehead, 1996). Persistence of T. juncea plants at populations may be aided by the clumping nature of plants, the ability to produce ramets and the ability to set seed from self pollen (although possibly only through rare autogamous events).
While most of the T. juncea populations are fragmented and some in a degraded state, many of the populations had a diversity of native bees (including buzz pollinating species) and a diversity of plant species (including species requiring buzz pollination, such as Hibbertia spp.). Thus it is possible that the nectarless flowers of T. juncea, which have viable pollen for approx. 14 d, are seldom visited, but one visit during this time may be enough for successful pollination (and this is supported by single applications of pollen to glasshouse plants, see Fig. 2). Alternatively, autogamous pollination events may be more common in the wild, with wind to assist flowers to decant pollen from anther pores, than demonstrated by the breeding system studies here that were conducted in the glasshouse. This hypothesis has additional merit considering that, in Australia, glasshouse tomatoes (Lycospersicon cultivars), which have poricidal anthers, are hand self‐pollinated to increase fruit‐set, yet field‐grown tomatoes are not; growers relying primarily on wind to shake pollen from anther pores in the absence of commercial tomato‐pollinators (bumblebees) from mainland Australia (see Hogendoorn et al., 2000).
In support of this hypothesis is the evidence from other systems where Tetratheca flowers are rarely visited by pollinators. Tetratheca gunnii Hook.f., an endangered species in decline in Tasmania, requires cross‐pollination for seed‐set, but in the wild fruit : flower ratios were only 1 % (Potts and Barker, 1999). In Western Australia (WA), Bell noted that a common Tetratheca species produced few seed (D. Bell, pers. comm., 1999) and, although most of the Tremandraceae occur in WA, Brown et al. (1997) have no listings of floral visitors to Tetratheca, Platytheca or Tremandra in their WA database of animals visiting flowers. In NSW, pollinators of T. glandulosa Smith, a vulnerable shrub found from Sydney to Mangrove Mountain, have never been detected during set observation periods (C. Brown, pers. comm., July 2003). In Tasmania, Hingston (1999) has recorded Homalictus niveifrons Cockrell (or possibly H. megastigmus Cockrell) and a Lasioglossum species at flowers of common Tetratheca glandulosa, although visitors were uncommon. He noted these bees on other shrubs, however, and that the larger halictines (and the introduced Apis mellifera L. and Bombus terrestris L.) do not visit flowers of T. glandulosa (A. Hingston, pers. comm., 3 May 2001). In South Australia, Houston observed female Homalictus bees visiting the flowers of T. pilosa Labill. on 28 Oct. 1976 in Belair National Park, and on 7 Nov. 1976 in Warren National Park (WA), where bees were found to be carrying a mixture of pollen grains, mostly Tetratheca but with a significant proportion of Calytrix grains (T. Houston, unpub. res., pers. comm., April 2001). Armstrong (1979), in a review of pollination mechanisms of the Australian flora, does not mention Tetratheca in an extensive list of native pollinators and their preferred plants.
Homalictus are small native bees (approx. 5 mm long) that build vertical nests in the soil (Dollin, 2000). In Walker’s revision of Homalictus he records just one visitor to Tetratheca—Homalictus megastigmus—and lists Warren National Park as the locality (see above) (Walker, 1986). Homalictus megastigmus occurs in all states except the Northern Territory. Walker (1986) and Cardale (1993) list Acacia, Bursaria, Leucopogon, Eucalyptus and Leptospermum and Tetratheca as floral resources for Homalictus megastigmus.
The evidence in the literature suggests that a putative pollinator for T. juncea could be Homalictus megastigmus (or other Homalictus species) but that Homalictus megastigmus is a generalist in its floral needs. This suggests that habitats of T. juncea will need to contain a diversity of species – this is not unexpected as T. juncea is nectarless and any species of foraging bee will need to gather their nectar supplies from other species growing with T. juncea. Other native bees that occur in the study region and that could potentially pollinate T. juncea include species in Amegilla, Anthophora, Exoneura, Lasioglossum, Leioproctus and Lestis (possibly too large) and Nomia.
There has been much discussion about a widespread decline in pollinators (Buchmann and Nabhan, 1996; Kearns et al., 1998). The main controversy is in regard to the degree to which there is actual demonstrable evidence of severe pollination deficits (Thomson, 2001) or that the phenomenon is prevalent across all continents and pollinator types (Cane, 2001). Habitat fragmentation, however, is considered by many (e.g. Rathcke and Jules, 1993; Didham et al., 1996; Kearns et al., 1998) to be a major disruptor of reproductive processes. Furthermore, empirical studies are accruing (e.g. Lamont et al., 1993; Cunningham, 2000; Donaldson et al., 2002; Jacquemyn et al., 2003) to support this and the position taken by several workers (e.g. Kevan and Phillips, 2001; Thomson, 2001) is that there is no cause for complacency. Tetratheca species are particularly vulnerable to any (further?) disruption of pollination services because pollination events are already rare and plants need to co‐flower with nectar‐producing species. Moreover, most of Tremandraceae is threatened, suggesting additional vulnerability to yet unknown processes associated with decline or rarity.
The small population sizes often found in T. juncea may exacerbate pollination failure because bees may only pollinate this species when there is a large volume of flowers in an area (Sih and Baltus, 1987). This suggests that patch density will also be an important attribute of pollination success. Support was found for this here, where there was a strong positive relationship between foliage volume and fruit: flower ratios, although larger plants may be able to emphasize female fitness components (e.g. Sletvold, 2002).
For the poorly fecund populations of T. juncea and other species of Tetratheca (see above), the pollen‐dispensing mechanism involving poricidal anthers does not appear to be a successful trait. In addition, the potential limitations of a buzz pollinated system, as outlined by Larson and Barrett (1999), are further exacerbated by the presence of tapetal fluid which regulates the amount of pollen release. Thus, the evolutionary advantages of these pollen‐dispensing mechanisms may be unrealized in small populations or populations experiencing low pollinator visitation rates (see also Larson and Barrett, 1999; Machado and Lopes, 2000). Factors that would make the sites less suitable for native bee occupation might include, among others, an unsuitable floral species’ assemblage [e.g. a lack of nectar resources at the right time of year or, alternatively, rich alternative source(s) of pollen and nectar], a lack of nesting locations (e.g. through the build‐up of leaf litter on soil), extirpation of populations through inappropriate fire regimes (fires during peak flowering periods) and competition with introduced honeybees for nectar resources. These remain interesting areas to investigate.
In summary, T. juncea, a species with a buzz pollination system, but so far elusive pollinators, produced fertile fruit in 24 populations at levels much higher than glasshouse autogamy results, suggesting that either a pollen vector is still to be discovered and/or that field levels of autogamy are elevated above glasshouse conditions. Populations in the SW with elevated fruit production and populations in the SE with high fruit : flower ratios, may be profitable locations for further floral visitor monitoring. To augment this, genetic assays of outcrossing rates within populations would be informative, especially as the tendency for plants to form clumps may be indicative of a high potential for inbreeding.
ACKNOWLEDGEMENTS
We thank Robert Payne for generously sharing his knowledge; D. Mackay for illustrations and fieldwork; Roy Mackay for fieldwork; CMLR staff (Gillian Kopittke for field assistance); UNE staff (Paul Lisle); Kevin Stokes (The University of Newcastle); Paul Williams, Newstan Colliery Powercoal Pty Ltd; Russell Williams, Vales Point Power Station, Delta Electricity; Nancy and Les Cornish, Karuah; Terry Tame, Newcastle Botanic Gardens. Special thanks to Claire Brown, David Bell, Andrew Hingston, Terry Houston, David Mackay, Duncan Mackay, Tish Silberbauer, Wendy Potts and Robert Whelan for sharing information and/or discussions. This work was carried out under a NSW TSC Act (1995) Section 91 (2) certificate (SYZ/99/037) and was funded by ACARP (ACARP C8012). We thank ACARP for sponsorship, Australian Coal Research, Oceanic Coal Limited, Powercoal Pty Ltd and Coal Operations Australia Ltd for sponsorships.
Supplementary Material
Received: 14 May 2003; Returned for revision: 11 July 2003; Accepted: 2 September 2003 Published electronically: 8 October 2003
References
- ArmstrongJA.1979. Biotic pollination mechanisms in the Australian flora – a review. New Zealand Journal of Botany 17: 467–508. [Google Scholar]
- BartierF, Gross CL, Mulligan D, Bellairs S, Bowen, D.2001. Understanding the biology and ecology of a vulnerable plant species – a case study with Tetratheca juncea occurring over coal leases. ACARP Project C8012. University of Queensland, St Lucia, Qld. [Google Scholar]
- BoesewinkelFD.1999. Ovules and seeds of Tremandraceae. Australian Journal of Botany 47: 769–781. [Google Scholar]
- BriggsJD, Leigh JH.1996.Rare or threatened Australian plants. 1995 revised edn. CSIRO: Australia. [Google Scholar]
- BrownEM, Burbidge AH, Dell J, Edinger D, Hopper SD, Wills, RT.1997.Pollination in Western Australia. A database of animals visiting flowers. Handbook No. 15. W.A. Naturalists’ Club, Perth. [Google Scholar]
- BuchmannS.1983. Buzz pollination in angiosperms. In: Jones CE, Little RJ, eds. Handbook of experimental pollination biology. New York: Scientific and American Editions, 73–113. [Google Scholar]
- BuchmannSL, Nabhan GP.1996.The forgotten pollinators. Washington, DC: Island Press. [Google Scholar]
- CaneJH.2001. Habitat fragmentation and native bees: a premature verdict? Conservation Ecology 5 [online: http://www.consecol.org/vol5/iss1/art3]. [Google Scholar]
- CardaleJC.1993. Hymenoptera: Apoidea. In: Houston WWK, Maynard GV, eds. Zoological catalogue of Australia, Vol. 10. Canberra: AGPS. [Google Scholar]
- CookSA, Stanley RG.1960. Tetrazolium chloride as an indicator of pine pollen germinability. Silvae Genetica 9: 134–136. [Google Scholar]
- CunninghamSA.2000. Effects of habitat fragmentation on the reproductive ecology of four plant species in mallee woodland. Conservation Biology 14: 758–768. [Google Scholar]
- DidhamRK, Ghazoul J, Stork NE, Davis AJ.1996. Insects in fragmented forests: a functional approach. TREE 11: 255–260. [DOI] [PubMed] [Google Scholar]
- DollinA, Batley M, Robinson M, Faulkner B.2000.Native bees of the Sydney region. A field guide. An Australian Native Bee Research Publication, Sydney. [Google Scholar]
- DonaldsonJ, Nanni I, Zachariades C, Kemper J, Thompson JD.2002. Effects of habitat fragmentation on pollinator diversity and plant reproductive success in renosterveld shrublands of South Africa. Conservation Biology 16: 1267–1276. [Google Scholar]
- GrossCL.1990. The breeding systems of three co‐occurring legumes: Dillwynia hispida, Dillwynia uncinata and Pultenaea densifolia (Leguminosae, Papilionoideae). Australian Journal of Botany 38: 207–215. [Google Scholar]
- GrossCL.1993. The breeding system and pollinators of Melastoma affine (Melastomataceae): a pioneer shrub in tropical Australia. Biotropica 25: 468–474. [Google Scholar]
- GrossCL, Mackay D.1998. Honeybees reduce fitness in the pioneer shrub Melastoma affine (Melastomataceae). Biological Conservation 86: 169–178. [Google Scholar]
- HingstonAB.1999. Affinities between southern Tasmania plants in native bee visitor profiles. Australian Journal of Zoology 47: 361–384 [Google Scholar]
- HogendoornK, Steen Z, Schwarz MP.2000. Native Australian carpenter bees as a potential alternative to introducing bumble bees for tomato pollination in greenhouses. Journal of Apicultural Research 39: 67–74. [Google Scholar]
- JacquemynH, Brys R, Hermy M.2003. Patch occupancy, population size and reproductive success of a forest herb (Primula elatior) in a fragmented landscape. Oecologia 130: 617–625 [DOI] [PubMed] [Google Scholar]
- KearnsCA, Inouye DW, Waser NM.1998. Endangered mutualisms: the conservation of plant–pollinator interactions. Annual Review of Ecology and Systematics 29: 83–112. [Google Scholar]
- KevanPG, Phillips TP.2001. The economic impact of pollinator declines: an approach to assessing consequences. Conservation Ecology 5 [online: http//www.consecol.org/vol5/iss1/art8]. [Google Scholar]
- KingMJ, Buchmann SL.1996. Sonication dispensing of pollen from Solanum laciniatum flowers. Functional Ecology 10: 449–456. [Google Scholar]
- LamontB, Klinkhamer PWE, Klinkhamer P, Witkowski E.1993. Population fragmentation may reduce fertility to zero in Banksia goodii – a demonstration of the Allee effect. Oecologia 94: 446–450. [DOI] [PubMed] [Google Scholar]
- LarsonBMH, Barrett SCH.1999. The ecology of pollen limitation in buzz‐pollinated Rhexia virginica (Melastomataceae). Journal of Ecology 87: 371–381. [PubMed] [Google Scholar]
- MachadoIC, Lopes AV.2000.Souroubea guianensis Aubl.: quest for its legitimate pollinator and the first record of tapetal oil in the Marcgraviaceae. Annals of Botany 85: 705–711. [Google Scholar]
- PayneRJ.1998. Lake Macquarie Tetratheca juncea Conservation Management Plan Interim Report September 1998. A report prepared for Lake Macquarie City Council, NSW National Parks and Wildlife Service and BHP Pty Ltd. [Google Scholar]
- PayneRJ.2000. Lake Macquarie Tetratheca juncea Conservation Management Plan Final Report November 2000. A report prepared for Lake Macquarie City Council, NSW National Parks and Wildlife Service and BHP Pty Ltd. [Google Scholar]
- PottsWC, Barker P.1999.Tetratheca gunnii Flora Recovery Plan. Department of Primary Industries, Water and Environment, Hobart. [Google Scholar]
- RathckeBJ, Jules ES.1993. Habitat fragmentation and plant–pollinator interactions. Current Science 65: 273–277. [Google Scholar]
- SihA, Baltus M.1987. Patch size, pollinator behavior, and pollinator limitation in catnip. Ecology 68: 1679–1690. [DOI] [PubMed] [Google Scholar]
- SletvoldN.2002. Effects of plant size on reproductive output and offspring performance in the facultative biennial Digitalis purpurea Journal of Ecology 90: 958–966. [Google Scholar]
- SteinerKE.1993. Has Ixianthes (Scrophulariaceae) lost its special bee? Plant Systematics and Evolution 185: 7–16. [Google Scholar]
- SteinerKE, Whitehead V B.1996. The consequences of specialization for pollination in a rare South African shrub, Ixanthes retzioides (Scrophulariaceae). Plant Systematics and Evolution 201: 131–138. [Google Scholar]
- ThompsonJ.1976. A Revision of the Genus Tetratheca (Tremandraceae). Telopea 1(3), 139–215. [Google Scholar]
- ThompsonJ.1978.Flora of New South Wales. No. 111. Tremandraceae Sydney: NSW Department of Agriculture, 1–21. [Google Scholar]
- ThomsonJD.2001. Using pollination deficits to infer pollinator declines: can theory guide us? Conservation Ecology 5 [online: http://www.consecol.org/vol5/iss1/art6]. [Google Scholar]
- WalkerKL.1986. Revision of the Australian species of the genus Homalictus Cockerell (Hymenoptera: Halictidae). Memoirs Museum Victoria 47: 105–200. [Google Scholar]
- WalterKS, Gillett HJ, (eds)1998. 1997 IUCN Red List of Threatened Plants. Compiled by the World Conservation Monitoring Centre. IUCN (The World Conservation Union), Gland, Switzerland and Cambridge, UK. [Google Scholar]
- WilliamsNM, Minckley RL and Silveira FA.2001. Variation in native bee faunas and its implications for detecting community changes. Conservation Ecology 5 [online: http://www.consecol.org/vol5/iss1/art7]. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.