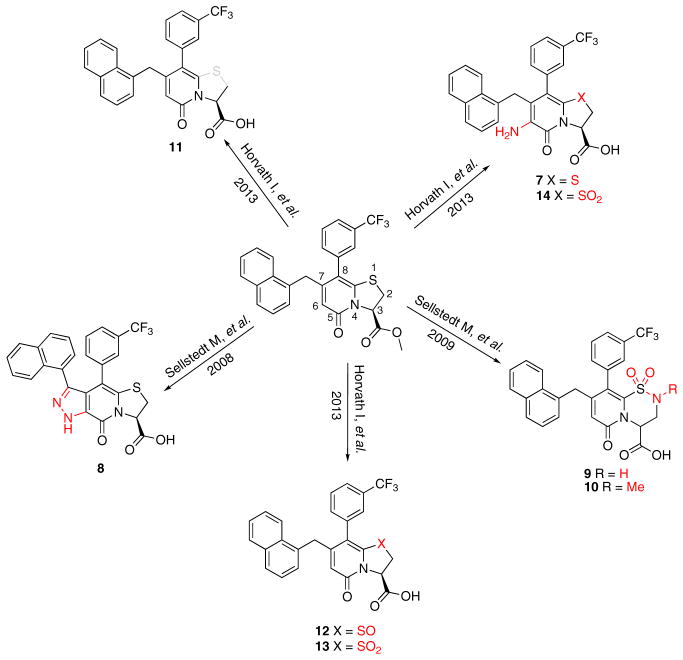
Figure 1. Compounds Synthesized to Analyze Central Fragment Alterations and Substituents.
With 1 as starting point, the peptidomimetic backbone was extended by introducing an amine in the pyridone ring resulting in compound 7. A rigidified tricyclic structure, compound 8, was included as well as analogs where the five-membered thiazolino group had been exchanged for six-membered sultams in compounds 9 and 10. Compound 11 is a desulfurized ring-opened analog and compounds 12 and 13 are analogs where the sulfur had been oxidized to the corresponding sulfoxide or sulfone. Compound 14 has both the extended peptidomimetic backbone and oxidized sulfur.
See also Table S1.