Figure 2.
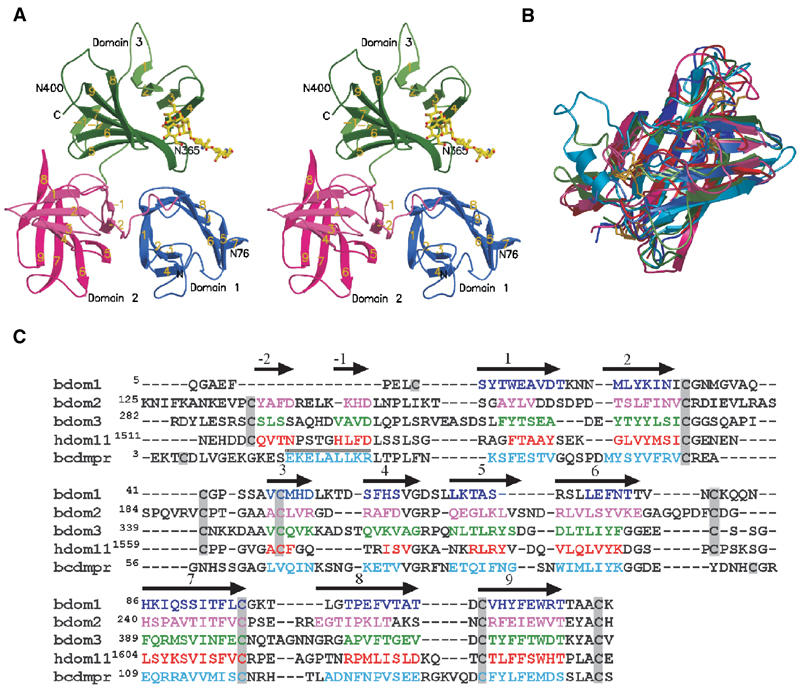
Structure of the three N-terminal domains of the bovine CI-MPR. (A) Stereo view of the ribbon diagram of the protein encoding domain 1 (blue), domain 2 (pink), and domain 3 (green). The oligosaccharide (yellow) attached to Asn76 of an adjacent crystallographic neighbor is shown. The position of the two glycosylated asparagine residues (Asn76 and Asn365) and that of the potential glycosylation site at Asn400 are indicated. The β-strands are labeled, showing the relative orientation of the domains to each other. (B) Overlay of the structures of the individual domains (domain 1, 2, and 3) along with those of the CD-MPR (cyan) and domain 11 (red) of the CI-MPR. Color schemes for domains 1–3 are the same as in panel A. The disulfide bridges are shown in gold. Note that the location of the first disulfide pair in domain 1 differs from that of domains 2, 3, and 11. (C) Structure-based sequence alignment of domains 1–3 of the bovine CI-MPR, domain 11 of the human CI-MPR, and the bovine CD-MPR. Secondary structure elements are indicated above the sequence, with arrows indicating β-strands and the cylinder indicating the single α-helix present in the CD-MPR. β-Strands of the β-barrel in each domain are sequentially numbered with strands in the preceding linker region as -1and -2. Residues found in the secondary structure elements are indicated by colored text. Cysteine residues are boxed in gray.
