Figure 6.
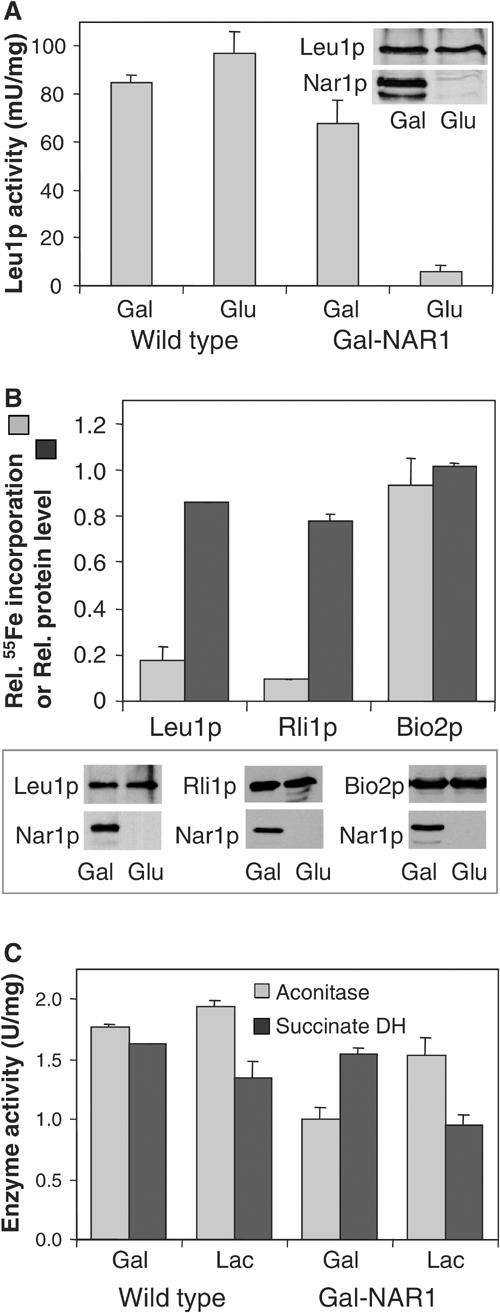
Nar1p is required for maturation of cytosolic, but not of mitochondrial, Fe/S proteins. (A) Wild-type and Gal-NAR1 cells were grown in minimal medium supplemented with galactose (Gal) or glucose (Glu). The enzyme activity of the cytosolic isopropylmalate isomerase (Leu1p) was estimated in total cell extracts. The levels of Leu1p and Nar1p in Gal-NAR1 cells were measured by immunoblot analysis (inset). (B) Gal-NAR1 cells were grown in iron-poor medium supplemented with galactose or glucose. Cells were transformed with high-copy plasmids carrying the genes for an HA-tagged version of cytosolic Rli1p or for mitochondrial Bio2p. Radiolabelling with 55Fe, preparation of cell extracts and immunoprecipitation of the Fe/S protein of interest were performed as in Figure 5. For Leu1p, the endogenous protein was analysed. Results are given as the ratio of 55Fe incorporation in Nar1p-depleted (glucose-grown) cells and that in Nar1p-expressing (galactose-grown) cells, corrected for a small background (<2% of total signal of galactose-grown cells). Protein levels of the indicated proteins were determined by immunoblot analysis (lower panel) and quantified by densitometry (upper panel). (C) The enzyme activities (in U per mg of mitochondrial protein) of aconitase and succinate dehydrogenase (DH) were assayed in mitochondria isolated from wild-type or Gal-NAR1 cells after growth in minimal medium containing galactose or lactate (Lac).
