Abstract
Dabigatran is an oral anticoagulant that is increasingly used for atrial fibrillation (AF). Presently, many authorities state that routine laboratory coagulation monitoring is not required. However, data have recently been published demonstrating that higher trough plasma dabigatran concentrations are associated with lower thromboembolic and higher haemorrhagic event rates. Using these data, we simulate a range of AF patients with varying risks for these events and derive a target range of trough plasma dabigatran concentrations (30–130 μg l−1). Finally, we propose that a conventional screening coagulation assay, the thrombin time (TT), can be used to discern whether or not patients are within this range of dabigatran concentrations.
Keywords: atrial fibrillation, blood coagulation, dabigatran, dosing, monitoring
Introduction
Dabigatran is an orally administered direct thrombin inhibitor used for the prevention of thromboembolism. It is a widely used anticoagulant for atrial fibrillation (AF) 1–6. It is often stated that dabigatran therapy does not require laboratory coagulation monitoring because it has a predictable dose–response relationship 7–10. These assertions have been questioned on the grounds that the reported population variability in dabigatran clearance is in the order of 50% (coefficient of variation, CV) even after accounting for differences in renal function between individuals 11. Notably, this CV is greater than that reported for S-warfarin clearance 12,13. In addition, therapeutic anticoagulation requires a balance between the risk of clotting and the risk of bleeding due to the innate variability and sensitivity of the coagulation network. It has therefore been proposed that dose-adjustment according to clotting times in the individual patient should be the standard of care when using any anticoagulant 12.
In this review, we begin with some background on dabigatran pharmacokinetics and pharmacodynamics, and construct a target trough dabigatran plasma concentration range using data from the published literature. We then discuss the use of coagulation assays to monitor dabigatran therapy. Finally, we make a case for using the conventional thrombin time (TT) to guide dabigatran dosing, discuss limitations of the present proposal and outline future work that may be needed.
Dabigatran pharmacokinetic and pharmacodynamic considerations
Dabigatran is a highly polar compound that is administered orally as an inactive prodrug, dabigatran etexilate 14,15. Dabigatran etexilate has a reported oral availability of about 7% 16, a value that is decreased by reduced stomach acidity 17,18 and by increased intestinal P-glycoprotein function (Figure 1) 19. Following absorption, dabigatran etexilate is metabolized by esterases, including carboxylesterase 1 (CES1), via the intermediate metabolites BIBR 951 and BIBR 1087, to the active metabolite, dabigatran 16,20. BIBR 951 is thought to have activity against thrombin, whereas BIBR 1087 is said to be inactive 21. The metabolism from dabigatran etexilate to dabigatran appears to be rapid and virtually complete, with the combined plasma AUC(0,∞) for the prodrug and its intermediate metabolites being less than 0.4% of the plasma dabigatran AUC(0,∞) in healthy volunteers 21,22. Therefore the contribution of BIBR 951 to drug activity can be considered insignificant 16.
Figure 1.
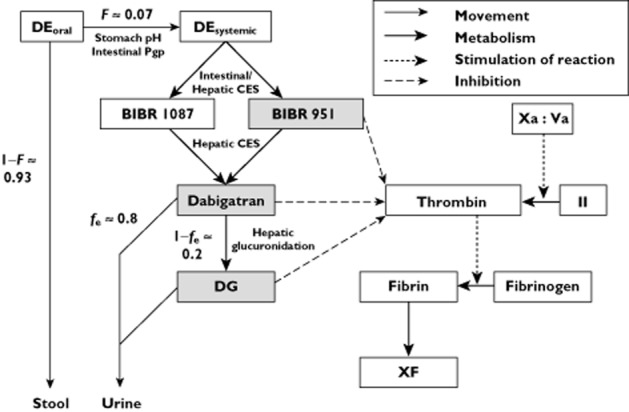
An abbreviated pharmacokinetic/pharmacodynamic model of dabigatran. DE, dabigatran etexilate; F, oral availability; fe, fraction excreted unchanged in urine; CES, carboxylesterase enzyme; DG, dabigatran glucuronides; Xa:Va, factor Xa:factor Va complex; II, factor II; XF, cross-linked fibrin. Grey boxes indicate reported thrombin inhibitors 68,21,23,69
Dabigatran itself is renally cleared, with a high fraction excreted unchanged in urine (fe) of around 0.8 16. Unlike its prodrug dabigatran etexilate, dabigatran is not known to be a substrate of P-glycoprotein. Plasma dabigatran concentrations are therefore not expected to be affected by P-glycoprotein function at the renal tubules 14. Glucuronidation appears to be responsible for the remainder of dabigatran clearance 16. Data from healthy volunteers suggest that the glucuronides are eliminated renally 16.
The principal measurable metabolite resulting from dabigatran glucuronidation is 1-O-acylglucuronide. The other glucuronides (2-O, 3-O and 4-O-acylglucuronide) were reported to be below the limit of detection in plasma samples taken up to 6 h following either oral dabigatran etexilate or intravenous dabigatran administration 16. The dabigatran glucuronides are all thought to be active against thrombin because substitution of glucuronic acid for the carboxylate functional group of dabigatran, does not interfere with the thrombin binding site 23. Dabigatran 1-O-acylglucuronide increases activated partial thromboplastin time (aPTT) in a dose-dependent manner to the same degree as dabigatran itself, for concentrations up to around 1000 nmol l−1 for either compound 23. To put this into context, the 90th percentile of peak plasma dabigatran concentrations observed in the RE-LY trial from over 9000 patients treated with dabigatran etexilate was around 700 nmol l−1 1,24,25. Therefore, for clinical purposes, dabigatran 1-O-acylglucuronide can be considered to have the same potency and efficacy as dabigatran itself, and the anticoagulant effect will depend on the combined AUC values of these compounds.
The magnitude of anticoagulation in an individual patient treated with dabigatran etexilate is determined by (i) the concentrations of active compounds at the site of action, including BIBR 951, dabigatran and its glucuronides and (ii) the concentrations and innate sensitivity of coagulation network constituents, including factor II and fibrinogen (Figure 1). Therefore, these will influence an individual’s risk of thromboembolic or haemorrhagic events following exposure to dabigatran etexilate.
A target dabigatran concentration range for AF treatment?
In this section, we examine relevant published data from individuals with AF given dabigatran etexilate regarding plasma dabigatran concentrations and thromboembolic and haemorrhagic events. Using these, we generate a proposed target plasma dabigatran concentration range.
Reilly et al. recently examined the relationships between dabigatran concentrations and thromboembolic and haemorrhagic events 25. These data came from the RE-LY trial, which randomized individuals with AF to warfarin, with dosing targeted at an International Normalized Ratio (INR) of 2–3, or to dabigatran etexilate with fixed dosing that was not guided by coagulation monitoring 1. There were two dabigatran etexilate arms to which individuals were randomly assigned, including 110 mg twice daily and 150 mg twice daily, with median follow-up of 2.0 years. After 1 month of maintenance dosing, a trough dabigatran concentration was collected from each individual, 10–16 h after the previous dose of dabigatran etexilate. The 10th to 90th percentiles of trough steady-state dabigatran concentrations for the 110 mg and 150 mg dabigatran etexilate arms were 28–155 and 40–215 μg l−1, respectively. The report by Reilly et al. demonstrated that higher dabigatran trough concentrations at steady-state were associated with lower rates of ischaemic stroke/systemic embolic event (SEE) and higher rates of major bleeding. They generated logistic regression curves to quantify the rates of these adverse events for an index 72-year-old male with non-valvular AF, diabetes mellitus and previous ischaemic stroke over the dabigatran trough concentration range of 10–300 μg l−1 (21–636 nmol l−1). The index 72-year-old male reflects the mean age and thromboembolic risk of the population studied in the RE-LY study 1.
The logistic regression curves, defined by Reilly et al., can be used to define a target trough concentration range that could guide dabigatran etexilate therapy in the setting of AF 12,26,27. We digitized these lines of best fit using Engauge Digitizer (version 4.1, http://sourceforge.net). The resulting data for trough concentrations vs. (i) ischaemic stroke/SEE risk and (ii) major bleeding risk were each fitted to a two phase exponential decay equation, with r2 > 0.999 for both risk equations (GraphPad Prism version 6.03, GraphPad Software, La Jolla, CA, USA, http://www.graphpad.com). Each equation was adjusted by weighting relevant adverse events according to the hazard ratios for death, relative to an ischaemic stroke. We used the event weight values generated by Eikelboom et al., which were based on data from the RE-LY study 28. It should be noted that the subtypes of events contributing to each of (i) ischaemic stroke/SEE and (ii) major bleeding, are not uniformly weighted. For example, both haemorrhagic stroke and extracranial bleeding were counted as major bleeding events, but had weights of 3.23 and 0.63, respectively. Thus, from the literature, we extracted the numbers of subtypes of events contributing to ischaemic stroke/SEE and major bleeding in the RE-LY study 1,29,30. Using these data, we generated the weight-adjusted risk equations for stroke/SEE and major bleeding (see Appendix for the equations).
We then generated a combined weight-adjusted risk equation by adding these equations together (see Appendix). The resulting plot is shown in Figure 2. The trough concentration (∼30 μg l−1) associated with the lowest combined weight-adjusted risk on our plot is relatively low compared with the observed trough concentrations reported for the two dabigatran etexilate dose rates in the RE-LY study. This reflects the finding by Reilly et al. that the thromboembolic risk declined steeply at low concentrations, with little additional advantage from higher concentrations 25.
Figure 2.
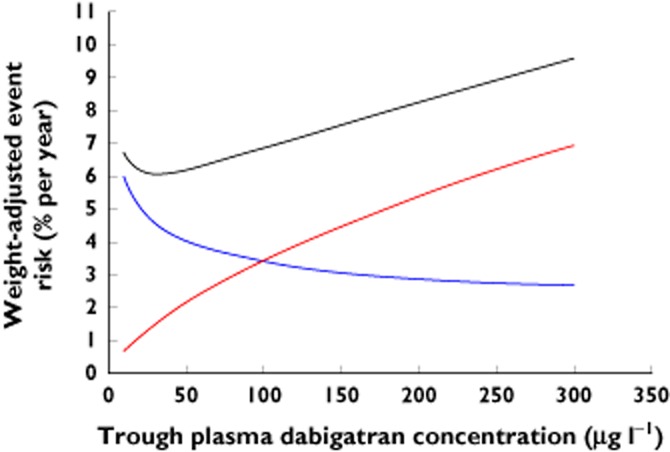
Weight-adjusted event risk curves for 72-year-old male with AF, diabetes mellitus and prior stroke (CHA2DS2-VASc = 4, HAS-BLED = 2). Black line, combined risk; blue line, stroke/systemic embolic event risk; red line, major bleeding risk
A more widely applicable target trough concentration range may be fashioned using standardized methods for assessing the balance of adverse event risks associated with anticoagulation in individuals with non-valvular AF 31–35. Of these, the CHA2DS2-VASc and HAS-BLED scoring systems are the most widely accepted methods for estimating an individual’s ischaemic stroke and major bleeding risk, respectively 36. The 2012 European Society of Cardiology guidelines for AF management state that patients with CHA2DS2-VASc ≥1 should routinely be anticoagulated 37. While Reilly et al. provide some data relating event rates in RE-LY to these two scoring systems, the CHA2DS2-VASc and HAS-BLED data are amalgamated into three (0–1, 2, >2) and two (0–1, >1) risk categories, respectively 25. Greater detail is available from previous studies of anticoagulation for non-valvular AF, including those involving ximelagatran, an earlier oral direct thrombin inhibitor, and warfarin (Table 1) 38,39. We calculate that the index 72-year-old male reported by Reilly et al. would have CHA2DS2-VASc and HAS-BLED scores of 4 and 2, respectively. By anchoring to this 72-year-old male, we proportionally adjusted our weight-adjusted risk equations for a range of individuals with a variety of combinations of CHA2DS2-VASc and HAS-BLED scores to explore the range of trough concentrations associated with the lowest combined weight-adjusted risk. Examples involving ‘extreme’ scores are presented in Figure 3. Figure 3A is consistent with the notion that patients with a high risk of major bleeding may not derive any benefit from dabigatran exposure, despite a moderate risk of stroke/SEE. For patients with non-valvular AF deemed to derive some benefit from dabigatran etexilate, Figures 3B and C suggest that the general target trough range is 30–130 μg l−1. Targeting dosing according to measured trough plasma dabigatran concentrations may therefore be a useful approach.
Table 1.
| CHA2DS2-VASc total score* | Thromboembolic risk (% per year) |
|---|---|
| 1 | 0.46 |
| 2 | 0.78 |
| 3 | 1.16 |
| 4 | 1.43 |
| 5 | 2.42 |
| 6 | 3.54 |
| 7 | 3.44 |
| 8 | 2.41 |
| 9 | 5.47 |
| HAS-BLED total score† | Major bleeding risk (% per year) |
|---|---|
| 0 | 1.2 |
| 1 | 2.8 |
| 2 | 3.6 |
| 3 | 6.0 |
| 4 | 9.5 |
| 5 | 7.5 |
| 6 | 0.0 |
CHA2DS2-VASc score (total 0–9 points) based on: 1 point for each of congestive heart failure/left ventricular dysfunction, hypertension, diabetes mellitus, vascular disease, female gender, age 65–74 years; 2 points for each of age ≥75 years, previous stroke/transient ischaemic attack/thromboembolism 32. A total score of 0 is not included as such individuals should not routinely be anticoagulated 37. Few individuals had a total score >6 37.
HAS-BLED score (total 0–9 points) based on: 1 point for each of hypertension, renal dysfunction, hepatic dysfunction, previous stroke, bleeding history/predisposition, labile INR, age >65 years, co-administered antiplatelet drug, alcohol excess 34. Few individuals had total score >4, and none had a total score >6 34.
Figure 3.
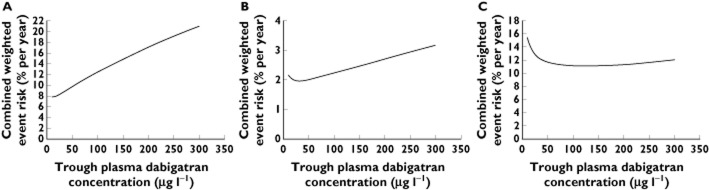
Combined weight-adjusted risk curves for: (A) CHA2DS2-VASc = 4, HAS-BLED = 4 (concentration associated with lowest combined risk ≤10 μg l−1); (B) CHA2DS2-VASc = 1, HAS-BLED = 0 (30 μg l−1); (C) CHA2DS2-VASc = 6, HAS-BLED = 2 (130 μg l−1)
Measuring dabigatran concentrations
While brief descriptions of plasma dabigatran assays have been reported in several clinical papers 16,18,19,21,22,40–43, only Delavenne et al. have published a comprehensive dabigatran assay method detailing the development and validation procedures in accordance with FDA guidelines 44. However, the mass spectrometry-based method presented by Delavenne et al. did not include quantification of the dabigatran glucuronides, which, as discussed above, contribute towards the anticoagulation effect. This becomes particularly relevant if the plasma concentrations of the glucuronides are significant relative to the concentration of dabigatran itself.
Reports of the contribution of the dabigatran glucuronides to total active drug-related exposure in plasma from dabigatran etexilate range from 10 to 35% 16,18,21,22,40,41, with no apparent influence of mild to severe renal 22 or moderate hepatic impairment 21. The range is only slightly narrower (15–35%) when the reports are restricted to those involving healthy young individuals who were administered a single dabigatran etexilate dose of 150 mg 18,21,22,41. However, the variability of the contribution of the glucuronides to overall drug exposure has been reported to be low in healthy young individuals, as reflected by a coefficient of variation (CV) of 8–10% 40. Based on this CV, for example, a mean contribution of 35% would have a 95% CI of 28, 42%. These findings suggest there is only modest biological variation in the contribution of the glucuronides to total active drug-related exposure. Instead, the reported variability (10–35%) most likely stems from inaccuracy in the measurement of the glucuronides.
Several sources of inaccuracy in the measurement of the glucuronides are possible. For example, the glucuronides may be unstable during sample storage 16. This is difficult to establish as the glucuronides were not assayed directly in any of the reported studies 16,18,21,22,40,41. Instead, these were inferred from the difference in plasma dabigatran concentrations before and after alkalinization (hydrolyzing the glucuronides to dabigatran) 16,18,21,22,40. In the RE-LY study, the reported dabigatran concentrations came from the measurement of dabigatran in alkalinized samples 24,25. It has been reported that 1-O-acylglucuronide spontaneously degrades in a pH 7.4 aqueous buffer at 37°C with an apparent in vitro half-life of 1 h 23. The stability of dabigatran under alkaline conditions is unclear 45. Beyond brief descriptions within clinical papers, we are not aware of any published method paper for a dabigatran assay that includes quantification of the glucuronides in human plasma.
Coagulation assays and dabigatran
Given the uncertainty surrounding the dabigatran assay, as well as its limited availability, the coagulation assays are attractive alternative biomarkers to guide dabigatran etexilate dosing. Coagulation assays are expected to reflect inter-individual variability in both dabigatran pharmacokinetics and pharmacodynamics, i.e. active drug concentrations and the endogenous coagulation network. A number of studies have examined the relationship between plasma dabigatran concentrations and a range of coagulation assays 40,46–54. These include the conventional screening assays, such as the INR, activated partial thromboplastin time (aPTT) and thrombin time (TT), and the less widely available assays, such as the ecarin clotting time (ECT) and the dilute thrombin time (dTT).
Of the conventional assays, the INR and aPTT are insensitive at lower plasma dabigatran concentrations, whereas the TT has been described as being excessively sensitive 48,49,51,55. Data from our research at Christchurch Hospital, which includes 52 individuals who provided 120 blood samples following at least 10 days of oral dabigatran etexilate at a constant dose rate, reflect these conclusions 56. All our dabigatran-treated individuals had TT values well above our ‘normal’ reference range of 18–28 s, with the lowest value being 82 s (Figure 4). Further, plasma dabigatran concentrations above 90 μg l−1 were consistently associated with TT values above the maximum reported time of 300 s.
Figure 4.
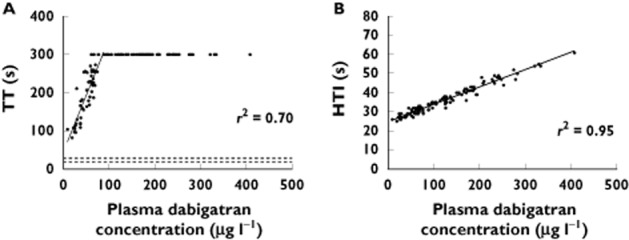
Correlation between plasma dabigatran concentrations and (A) TT and (B) HTI. For TT, the maximum reported time was 300 s, and TT > 300 s have been set to 300 s (no reported TT value was actually 300 s). Solid lines represent the lines of best fit. Dashed lines represent the reference range at our institution, Christchurch Hospital. r2 values are for the line of best fit, which for the TT data do not include those values with TT > 300 s. Data from 52 individuals on dabigatran etexilate providing 120 blood samples at steady-state [56]
The dTT was developed to compensate for the sensitivity of the TT in the presence of direct thrombin inhibitors 57. The dTT involves dilution of the test plasma sample with normal pooled plasma. At least two published versions of the dTT have been developed specifically for dabigatran 50,52, including a commercial assay (Hemoclot® Thrombin Inhibitor, HTI, Hyphen BioMed, Neuville-sur-Oise, France). r2 values in excess of 0.90 have consistently been reported between HTI times and plasma dabigatran concentrations 47,48,51–53, which is confirmed by our data (Figure 4B). Further, the HTI assay can be used to quantify indirectly ‘total’ dabigatran plasma concentration by using calibrators from the manufacturers of the HTI assay, which consist of plasma samples containing known concentrations of dabigatran.
The dabigatran concentration–clotting time relationships outlined above will vary depending on the clot-activating reagent, and the analyzer instrument used for the coagulation assays 48,51. Nonetheless, at first glance, the HTI assay appears to be suitable for monitoring patients on dabigatran. However, there is a potentially important caveat regarding the HTI assay and the dTT tests in general. As a consequence of the dilution with normal pooled plasma, the impact of inter-individual differences in plasma fibrinogen concentrations on the clotting time is diminished (Figure 1) 51. Therefore, the dTT tests are more correctly considered as tests for quantifying the total amount of thrombin inhibitors resulting from dabigatran etexilate administration (including BIBR 951, dabigatran and the dabigatran glucuronides), rather than as coagulation assays that account for both active drug concentrations and the individual patient’s endogenous coagulation network. The TT, on the other hand, is more representative of the interplay of active drug concentrations and clotting factors, and given its wide availability deserves further consideration as a marker for guiding dabigatran dosing.
The thrombin time for monitoring dabigatran effect and guiding dosing
The potential utility of the TT in comparison with the HTI assay is illustrated in Figure 5, which shows the Christchurch data restricted to dabigatran concentrations < 90 μg l−1. Data for dabigatran concentrations ≥90 μg l−1 were excluded from this plot as the TT values were consistently >300 s (our maximum reported TT). For the two data points indicated with arrows in Figure 5A, it can be postulated that the TT of 254 s represents a greater degree of anticoagulation than the point with a TT of 155 s, even though the dabigatran concentrations are 44 and 45 μg l−1, respectively. The corresponding points on the HTI in Figure 5B have results of 29 and 32 s, respectively. Note that both TT and HTI assays have a CV of <11% at our laboratory 56. Given that the dabigatran glucuronides (amounting to 10–35% of total active drug exposure) were not measured by our dabigatran assay, the total active dabigatran concentrations would be 11–54% higher than the measured dabigatran concentrations, to account for the dabigatran glucuronides. In this case, the ‘total’ dabigatran concentration above which our TT assay consistently returned values >300 s would be ≥100 μg l−1. Thus, it appears that our TT assay gives quantifiable results at dabigatran concentrations ≤100 μg l−1. While this is less than 130 μg l−1, the upper limit of the target dabigatran concentration derived in the section ‘A target dabigatran concentration range for AF treatment?’ above, an inspection of Figure 3C indicates that combined weight-adjusted risk for 100 μg l−1is minimally higher than 130 μg l−1. Using the line of best fit in Figure 5A, the target trough plasma dabigatran concentration of 30–100 μg l−1 corresponds to a TT range of 130–300 s.
Figure 5.
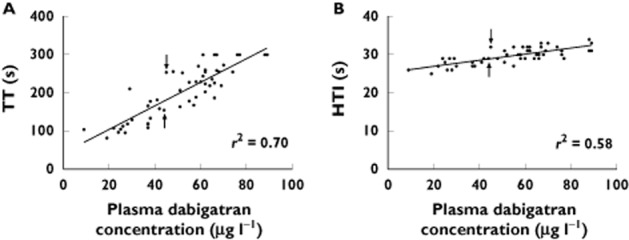
Correlation between plasma dabigatran concentrations <90 μg l−1 and (A) TT and (B) HTI. For TT graph, TT > 300 s set to 300 s. Solid lines represent the lines of best fit. r2 values are for the line of best fit, which for the TT data do not include those values with TT > 300 s. Arrows indicate a selected pair of blood samples (see text, section ‘The thrombin time for monitoring dabigatran effect and guiding dosing’)
It has been argued that a ‘rapid, widely available, specific and sensitive assay’ is required for monitoring dabigatran 51. We believe the TT goes a long way towards fulfilling the stated requirements. Individuals who might benefit from monitoring the anticoagulation effect include those who are about to undergo invasive procedures, have suffered a thromboembolic or haemorrhagic event or are suspected of non-adherence 7–9,58–60. Further, other individuals may also benefit from laboratory coagulation evaluation, including those for whom dabigatran etexilate treatment is currently contraindicated as a result of pharmacokinetic drug interactions 19, and those for whom the covariates of dabigatran concentrations are relevant (Table 2) 59–62.
Table 2.
Magnitude of effect of covariates on dabigatran plasma concentrations
| Covariate | Mean exposure ratio (90% CI)* |
|---|---|
| Stomach acidity | |
| Pantoprazole [18] | 0.80 (0.67, 0.95) |
| Intestinal P-glycoprotein function | |
| Verapamil [42]† | 1.71 (1.34, 2.15) |
| Rifampicin [19]‡ | 0.33 (0.27, 0.41) |
| rs4148738 [20]¶ | 1.12 (1.08, 1.17) |
| Intestinal/hepatic CES1 function | |
| rs2244613 [20]†† | 0.85 (0.81, 0.90) |
| Renal impairment [22] | |
| Mild | 1.50 (0.78, 2.90) |
| Moderate | 3.15 (1.63, 6.08) |
| Severe | 6.31 (3.54, 11.25) |
| Patient adherence | Not available |
| Opening the dabigatran etexilate capsule prior to administration [8] | 1.75 |
| Prolonged exposure of the capsule to humidity [70] | Not available |
This represents the mean ratio (90% CI) of the AUC(0,∞) of individuals with the covariate to healthy controls without the covariate, or for polymorphisms, the mean ratio (95% CI) of either peak or trough concentrations of single allele carriers to wildtype.
Other P-glycoprotein inhibitors (with reported mean AUC(0,∞) ratio) include: ketoconazole (2.5) 9; dronedarone (2.0) 71; amiodarone (1.6) 9; clarithromycin (1.5) 43; quinidine (1.5) 9; ticagrelor (1.5) [72] loading doses of clopidogrel (1.3) 41.
Other P-glycoprotein inducers include carbamazepine, phenytoin, phenobarbitone (exposure ratios have not been reported) 19.
The other ABCB1 polymorphism (with reported mean ratio of peak concentrations)is rs1045642 (1.08) 65.
Limitations of the present target trough TT proposal
There are several important limitations to our proposal. Ideally, a target TT range would be defined directly from events, but there are currently insufficient data in the public domain for such an analysis. The trough TT range of 130–300 s we propose reflects a trough plasma dabigatran range of 30–100 μg l−1. The foundation upon which this range is derived includes the veracity of a) drug concentration measurements, for both the RE-LY study (as discussed above) and the data from our observations and b) the relationship between the drug concentrations and events. A significant limitation of the analysis by Reilly et al. is that dabigatran concentration data largely came from blood samples taken 1 month following enrolment, whereas the majority of thromboembolic and haemorrhagic events occurred at least 12 months following enrolment 1,30. This time lag is likely to have contributed to the wide 95% CI of the lines of best fit of the logistic regression curves 25. Further, whilst biologically plausible, the relationship between events and plasma dabigatran concentrations can be questioned. In considering this, there are the data from the BISTRO II trial relating dabigatran concentrations to thrombotic and haemorrhagic rates in patients post-hip/knee joint replacement 63. Post-operatively, dabigatran etexilate was administered for a duration of 6–10 days and plasma concentrations were measured within the first 5 days. The duration of follow-up was 4–6 weeks. The time lag between blood samples for drug concentrations and events was thus shorter in the BISTRO II trial than in the RE-LY trial. Peak dabigatran concentrations at steady-state for the 1576 individuals administered dabigatran etexilate in BISTRO II were reported to be 48–271 μg l−1. Logistic regression curves correlating peak plasma dabigatran concentration with rates of deep vein thrombosis and bleeding indicate that concentrations around 50–100 μg l−1 were associated with the lowest combined adverse event risk. While these were peak rather than trough concentrations, they are consistent with the notion that the plasma dabigatran concentrations associated with the lowest combined risk of thrombotic and haemorrhagic events are relatively low compared with the total range of concentrations reported in both the RE-LY and BISTRO II trials 25,63.
We derived the range by simulating ‘extreme’ individuals (Figure 3), which is based on several assumptions. We converted the risk data for various CHA2DS2-VASc and HAS-BLED scores from warfarin/ximelagatran trials by anchoring to the index 72-year-old male described by Reilly et al. and applied these to dabigatran (Table 1). This assumes that the relationships between different scores from the warfarin/ximelagatran trials are relevant to dabigatran, e.g. that an individual with HAS-BLED = 4 has 9.5/3.6 times the major bleeding risk of an individual with a score of 2. Further, the ‘universal’ range, 30–100 μg l−1, may not be appropriate for all individuals, and instead an approach using individualized targets may be superior 64. However, a FDA analysis of the RE-LY trial data had findings similar to our own 65. We submit that our proposed target trough dabigatran concentrations and TT should be investigated further, and that our present proposal is merely a step towards finding an approach to dosing dabigatran etexilate that will ultimately improve patient outcomes.
Regarding patient outcomes, there may be little gain in terms of ischaemic stroke/SEE from using a targeted approach, given that well in excess of 90% of individuals in the RE-LY trial achieved trough concentrations over 30 μg l−1 25. Instead, the main anticipated benefit of our proposal is a reduction in major bleeding risk, which is relevant given that there is presently no readily available, rapid and convenient method of reversing the anticoagulant effects resulting from dabigatran etexilate 66.
Our proposed TT target range is specific to the TT assay we used, and its wider applicability is thus hindered by the lack of standardization of TT assays. How might others apply our proposed trough TT target range of 130–300 s, based on a target dabigatran concentration range of 30–100 μg l−1? Given that our reference range is 18–28 s, the range of the TT ratio based on the mid-range of 23 s is around 6–13. How does this conversion from plasma dabigatran concentrations to TT ratios compare with other studies using data from individuals given dabigatran? Using the plots of TT ratio vs. plasma dabigatran concentrations reported by Stangier et al. 40 and Hawes et al. 48, a range of 30–100 μg l−1 converts to TT ratios of 4–9 and 5–15, respectively. Given the widespread uptake of dabigatran etexilate, there may be an argument, ultimately, for standardization of the TT assays.
The problem of the ‘excessive’ sensitivity of the TT limits the amount of information available to the clinician from this test when, such as occurs at our laboratory, a result above the maximum reported time is only reported as the maximum time itself. How much higher is the true result? It has been suggested that, of the conventional screening coagulation assays, the INR and aPTT can be used in addition to the TT to distinguish between various degrees of high dabigatran exposure 48. Given the ready availability and rapid turnaround of these assays, it seems reasonable to use this combination of coagulation assays, especially when a TT above the maximum reported time is reported, or overdose is suspected.
Future work
Data from studies addressing the limitations will be important in progressing this proposal. Further analyses from the RE-LY study of clinical outcomes in relation to clotting times are anticipated 25,67. Coagulation results and plasma dabigatran concentrations from blood samples that are temporally proximate to relevant adverse clinical events may allow more accurate and precise delineation of the dose–response relationship.
If and when the above issues are addressed, and the target TT appropriately revised, dabigatran etexilate dosing guided by TT feedback should be tested in an interventional trial. If instead, outcomes are found to have a stronger correlation with plasma dabigatran concentrations than clotting times, a thorough examination of the assay methods for measuring the glucuronides, by comparing assays with and without sample alkalinization, should be carried out.
Conclusions
Oral anticoagulation using dabigatran etexilate in the setting of AF has achieved widespread global uptake, with impressive ‘real world’ results reflecting those achieved in the pivotal RE-LY trial 4,5,68. This is despite assertions that it is a drug with a narrow therapeutic index 55,58. Nonetheless, as pointed out by Duffull et al., ‘where it is possible to reduce the risk of bleeding or thrombosis … by taking reasonable actions, these actions should be taken’ 58. We believe monitoring the TT in patients treated with dabigatran etexilate, and using this feedback to adjust dosing, is a reasonable and potentially beneficial action.
Appendix
Equation 1 Ischaemic stroke/systemic embolic event weight-adjusted risk equation.
Equation 2 Major bleeding weight-adjusted risk equation.
Equation 3 Combined weight-adjusted risk equation.
D is the trough plasma dabigatran concentration at steady-state. All constants have been rounded to three significant figures for the purpose of presentation here.
Competing Interests
All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare no support from any organization for the submitted work, no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years and no other relationships or activities that could appear to have influenced the submitted work. MPD was a member of the Pharmaceutical Benefits Advisory Committee of Australia (PBAC) that considered an application for the funding of dabigatran and provided advice to the Australian government about funding dabigatran.
Paul K. L. Chin is a recipient of the Health Research Council of New Zealand Clinical Research Training Fellowship (2012–2014).
References
- Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, Pogue J, Reilly PA, Themeles E, Varrone J, Wang S, Alings M, Xavier D, Zhu J, Diaz R, Lewis BS, Darius H, Diener HC, Joyner CD, Wallentin L. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–1151. doi: 10.1056/NEJMoa0905561. [DOI] [PubMed] [Google Scholar]
- Connolly SJ, Wallentin L, Ezekowitz MD, Eikelboom J, Oldgren J, Reilly PA, Brueckmann M, Pogue J, Alings M, Amerena JV, Avezum A, Baumgartner I, Budaj AJ, Chen JH, Dans AL, Darius H, Di Pasquale G, Ferreira J, Flaker GC, Flather MD, Franzosi MG, Golitsyn SP, Halon DA, Heidbuchel H, Hohnloser SH, Huber K, Jansky P, Kamensky G, Keltai M, Kim SS, Lau CP, Le Heuzey JY, Lewis BS, Liu L, Nanas J, Omar R, Pais P, Pedersen KE, Piegas LS, Raev D, Smith PJ, Talajic M, Tan RS, Tanomsup S, Toivonen L, Vinereanu D, Xavier D, Zhu J, Wang SQ, Duffy CO, Themeles E, Yusuf S. The long-term multicenter observational study of dabigatran treatment in patients with atrial fibrillation (RELY-ABLE) study. Circulation. 2013;128:237–243. doi: 10.1161/CIRCULATIONAHA.112.001139. [DOI] [PubMed] [Google Scholar]
- Metcalfe S, Moodie P. National prescribing data for dabigatran. N Z Med J. 2012;125:97–105. [PubMed] [Google Scholar]
- Southworth MR, Reichman ME, Unger EF. Dabigatran and postmarketing reports of bleeding. N Engl J Med. 2013;368:1272–1274. doi: 10.1056/NEJMp1302834. [DOI] [PubMed] [Google Scholar]
- Sorensen R, Gislason G, Torp-Pedersen C, Olesen JB, Fosbol EL, Hvidtfeldt MW, Karasoy D, Lamberts M, Charlot M, Kober L, Weeke P, Lip GY, Hansen ML. Dabigatran use in Danish atrial fibrillation patients in 2011: a nationwide study. BMJ Open. 2013;3 doi: 10.1136/bmjopen-2013-002758. e002758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Holbrook AM, Simpson CS, Dowlatshahi D, Johnson AP. Prescribing patters of novel oral anticoagulants following regulatory approval for atrial fibrillation in Ontario, Canada: a population-based descriptive analysis. Can Med Assoc J Open. 2013;1:E115–119. doi: 10.9778/cmajo.20130032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ryn J, Stangier J, Haertter S, Liesenfeld KH, Wienen W, Feuring M, Clemens A. Dabigatran etexilate – a novel, reversible, oral direct thrombin inhibitor: interpretation of coagulation assays and reversal of anticoagulant activity. Thromb Haemost. 2010;103:1116–1127. doi: 10.1160/TH09-11-0758. [DOI] [PubMed] [Google Scholar]
- Gulseth MP, Wittkowsky AK, Fanikos J, Spinler SA, Dager WE, Nutescu EA. Dabigatran etexilate in clinical practice: confronting challenges to improve safety and effectiveness. Pharmacotherapy. 2011;31:1232–1249. doi: 10.1592/phco.31.12.1232. [DOI] [PubMed] [Google Scholar]
- Huisman MV, Lip GY, Diener HC, Brueckmann M, van Ryn J, Clemens A. Dabigatran etexilate for stroke prevention in patients with atrial fibrillation: resolving uncertainties in routine practice. Thromb Haemost. 2012;107:838–847. doi: 10.1160/TH11-10-0718. [DOI] [PubMed] [Google Scholar]
- Diener HC. Pro: ‘The novel oral anticoagulants should be used as 1st choice for secondary prevention in patients with atrial fibrillation’. Thromb Haemost. 2013;110:493–495. doi: 10.1160/TH13-04-0277. [DOI] [PubMed] [Google Scholar]
- Troconiz IF, Tillmann C, Liesenfeld KH, Schafer HG, Stangier J. Population pharmacokinetic analysis of the new oral thrombin inhibitor dabigatran etexilate (BIBR 1048) in patients undergoing primary elective total hip replacement surgery. J Clin Pharmacol. 2007;47:371–382. doi: 10.1177/0091270006297228. [DOI] [PubMed] [Google Scholar]
- Duffull SB. Is the ideal anticoagulant a myth? Expert Rev Clin Pharmacol. 2012;5:231–236. doi: 10.1586/ecp.12.18. [DOI] [PubMed] [Google Scholar]
- Wright DF, Al-Sallami HS, Duffull SB. Is the dose of dabigatran really more predictable than warfarin? Br J Clin Pharmacol. 2013;76:997–998. doi: 10.1111/bcp.12144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stangier J, Clemens A. Pharmacology, pharmacokinetics, and pharmacodynamics of dabigatran etexilate, an oral direct thrombin inhibitor. Clin Appl Thromb Hemost. 2009;15(Suppl. 1):9S–16S. doi: 10.1177/1076029609343004. [DOI] [PubMed] [Google Scholar]
- van Ryn J, Goss A, Hauel N, Wienen W, Priepke H, Nar H, Clemens A. The discovery of dabigatran etexilate. Front Pharmacol. 2013;4:12. doi: 10.3389/fphar.2013.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blech S, Ebner T, Ludwig-Schwellinger E, Stangier J, Roth W. The metabolism and disposition of the oral direct thrombin inhibitor, dabigatran, in humans. Drug Metab Dispos. 2008;36:386–399. doi: 10.1124/dmd.107.019083. [DOI] [PubMed] [Google Scholar]
- Stangier J, Eriksson BI, Dahl OE, Ahnfelt L, Nehmiz G, Stahle H, Rathgen K, Svard R. Pharmacokinetic profile of the oral direct thrombin inhibitor dabigatran etexilate in healthy volunteers and patients undergoing total hip replacement. J Clin Pharmacol. 2005;45:555–563. doi: 10.1177/0091270005274550. [DOI] [PubMed] [Google Scholar]
- Stangier J, Stahle H, Rathgen K, Fuhr R. Pharmacokinetics and pharmacodynamics of the direct oral thrombin inhibitor dabigatran in healthy elderly subjects. Clin Pharmacokinet. 2008;47:47–59. doi: 10.2165/00003088-200847010-00005. [DOI] [PubMed] [Google Scholar]
- Hartter S, Koenen-Bergmann M, Sharma A, Nehmiz G, Lemke U, Timmer W, Reilly PA. Decrease in the oral bioavailability of dabigatran etexilate after co-medication with rifampicin. Br J Clin Pharmacol. 2012;74:490–500. doi: 10.1111/j.1365-2125.2012.04218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pare G, Eriksson N, Lehr T, Connolly S, Eikelboom J, Ezekowitz MD, Axelsson T, Haertter S, Oldgren J, Reilly P, Siegbahn A, Syvanen AC, Wadelius C, Wadelius M, Zimdahl-Gelling H, Yusuf S, Wallentin L. Genetic determinants of dabigatran plasma levels and their relation to bleeding. Circulation. 2013;127:1404–1412. doi: 10.1161/CIRCULATIONAHA.112.001233. [DOI] [PubMed] [Google Scholar]
- Stangier J, Stahle H, Rathgen K, Roth W, Shakeri-Nejad K. Pharmacokinetics and pharmacodynamics of dabigatran etexilate, an oral direct thrombin inhibitor, are not affected by moderate hepatic impairment. J Clin Pharmacol. 2008;48:1411–1419. doi: 10.1177/0091270008324179. [DOI] [PubMed] [Google Scholar]
- Stangier J, Rathgen K, Stahle H, Mazur D. Influence of renal impairment on the pharmacokinetics and pharmacodynamics of oral dabigatran etexilate: an open-label, parallel-group, single-centre study. Clin Pharmacokinet. 2010;49:259–268. doi: 10.2165/11318170-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Ebner T, Wagner K, Wienen W. Dabigatran acylglucuronide, the major human metabolite of dabigatran: in vitro formation, stability, and pharmacological activity. Drug Metab Dispos. 2010;38:1567–1575. doi: 10.1124/dmd.110.033696. [DOI] [PubMed] [Google Scholar]
- Liesenfeld KH, Lehr T, Dansirikul C, Reilly PA, Connolly SJ, Ezekowitz MD, Yusuf S, Wallentin L, Haertter S, Staab A. Population pharmacokinetic analysis of the oral thrombin inhibitor dabigatran etexilate in patients with non-valvular atrial fibrillation from the RE-LY trial. J Thromb Haemost. 2011;9:2168–2175. doi: 10.1111/j.1538-7836.2011.04498.x. [DOI] [PubMed] [Google Scholar]
- Reilly PA, Lehr T, Haertter S, Connolly SJ, Yusuf S, Eikelboom JW, Ezekowitz MD, Nehmiz G, Wang S, Wallentin L. The effect of dabigatran plasma concentrations and patient characteristics on the frequency of ischemic stroke and major bleeding in atrial fibrillation patients in the RE-LY trial. J Am Coll Cardiol. 2014;63:321–328. doi: 10.1016/j.jacc.2013.07.104. [DOI] [PubMed] [Google Scholar]
- Kurnik D, Loebstein R, Halkin H, Gak E, Almog S. 10 years of oral anticoagulant pharmacogenomics: what difference will it make? A critical appraisal. Pharmacogenomics. 2009;10:1955–1965. doi: 10.2217/pgs.09.149. [DOI] [PubMed] [Google Scholar]
- Chin PK, Wright DF, Doogue MP, Begg EJ. Learning more from the dabigatran concentrations in RE-LY. J Am Coll Cardiol. doi: 10.1016/j.jacc.2013.11.069. doi: 10.1016/j.jacc.2013.11.069. [DOI] [PubMed] [Google Scholar]
- Eikelboom JW, Connolly SJ, Hart RG, Wallentin L, Reilly P, Oldgren J, Yang S, Yusuf S. Balancing the benefits and risks of 2 doses of dabigatran compared with warfarin in atrial fibrillation. J Am Coll Cardiol. 2013;62:900–908. doi: 10.1016/j.jacc.2013.05.042. [DOI] [PubMed] [Google Scholar]
- Connolly SJ, Ezekowitz MD, Yusuf S, Reilly PA, Wallentin L. Newly identified events in the RE-LY trial. N Engl J Med. 2010;363:1875–1876. doi: 10.1056/NEJMc1007378. [DOI] [PubMed] [Google Scholar]
- Eikelboom JW, Wallentin L, Connolly SJ, Ezekowitz M, Healey JS, Oldgren J, Yang S, Alings M, Kaatz S, Hohnloser SH, Diener HC, Franzosi MG, Huber K, Reilly P, Varrone J, Yusuf S. Risk of bleeding with 2 doses of dabigatran compared with warfarin in older and younger patients with atrial fibrillation: an analysis of the randomized evaluation of long-term anticoagulant therapy (RE-LY) trial. Circulation. 2011;123:2363–2372. doi: 10.1161/CIRCULATIONAHA.110.004747. [DOI] [PubMed] [Google Scholar]
- Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA. 2001;285:2864–2870. doi: 10.1001/jama.285.22.2864. [DOI] [PubMed] [Google Scholar]
- Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the Euro Heart Survey on atrial fibrillation. Chest. 2010;137:263–272. doi: 10.1378/chest.09-1584. [DOI] [PubMed] [Google Scholar]
- Gage BF, Yan Y, Milligan PE, Waterman AD, Culverhouse R, Rich MW, Radford MJ. Clinical classification schemes for predicting hemorrhage: results from the National Registry of Atrial Fibrillation (NRAF) Am Heart J. 2006;151:713–719. doi: 10.1016/j.ahj.2005.04.017. [DOI] [PubMed] [Google Scholar]
- Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest. 2010;138:1093–1100. doi: 10.1378/chest.10-0134. [DOI] [PubMed] [Google Scholar]
- Fang MC, Go AS, Chang Y, Borowsky LH, Pomernacki NK, Udaltsova N, Singer DE. A new risk scheme to predict warfarin-associated hemorrhage: the ATRIA (Anticoagulation and Risk Factors in Atrial Fibrillation) Study. J Am Coll Cardiol. 2011;58:395–401. doi: 10.1016/j.jacc.2011.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lip GY. Stroke and bleeding risk assessment in atrial fibrillation: when, how, and why? Eur Heart J. 2013;34:1041–1049. doi: 10.1093/eurheartj/ehs435. [DOI] [PubMed] [Google Scholar]
- Camm AJ, Lip GY, De Caterina R, Savelieva I, Atar D, Hohnloser SH, Hindricks G, Kirchhof P Guidelines ESCCfP. 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur Heart J. 2012;33:2719–2747. doi: 10.1093/eurheartj/ehs253. [DOI] [PubMed] [Google Scholar]
- Lip GY, Frison L, Halperin JL, Lane DA. Identifying patients at high risk for stroke despite anticoagulation: a comparison of contemporary stroke risk stratification schemes in an anticoagulated atrial fibrillation cohort. Stroke. 2010;41:2731–2738. doi: 10.1161/STROKEAHA.110.590257. [DOI] [PubMed] [Google Scholar]
- Lip GY, Frison L, Halperin JL, Lane DA. Comparative validation of a novel risk score for predicting bleeding risk in anticoagulated patients with atrial fibrillation: the HAS-BLED (Hypertension, Abnormal Renal/Liver Function, Stroke, Bleeding History or Predisposition, Labile INR, Elderly, Drugs/Alcohol Concomitantly) score. J Am Coll Cardiol. 2011;57:173–180. doi: 10.1016/j.jacc.2010.09.024. [DOI] [PubMed] [Google Scholar]
- Stangier J, Rathgen K, Stahle H, Gansser D, Roth W. The pharmacokinetics, pharmacodynamics and tolerability of dabigatran etexilate, a new oral direct thrombin inhibitor, in healthy male subjects. Br J Clin Pharmacol. 2007;64:292–303. doi: 10.1111/j.1365-2125.2007.02899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartter S, Sennewald R, Schepers C, Baumann S, Fritsch H, Friedman J. Pharmacokinetic and pharmacodynamic effects of comedication of clopidogrel and dabigatran etexilate in healthy male volunteers. Eur J Clin Pharmacol. 2013;69:327–339. doi: 10.1007/s00228-012-1304-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartter S, Sennewald R, Nehmiz G, Reilly P. Oral bioavailability of dabigatran etexilate (Pradaxa(R)) after co-medication with verapamil in healthy subjects. Br J Clin Pharmacol. 2013;75:1053–1062. doi: 10.1111/j.1365-2125.2012.04453.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delavenne X, Ollier E, Basset T, Bertoletti L, Accassat S, Garcin A, Laporte S, Zufferey P, Mismetti P. A semi-mechanistic absorption model to evaluate drug–drug interaction with dabigatran: application with clarithromycin. Br J Clin Pharmacol. 2013;76:107–113. doi: 10.1111/bcp.12055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delavenne X, Moracchini J, Laporte S, Mismetti P, Basset T. UPLC MS/MS assay for routine quantification of dabigatran – a direct thrombin inhibitor – in human plasma. J Pharm Biomed Anal. 2012;58:152–156. doi: 10.1016/j.jpba.2011.09.018. [DOI] [PubMed] [Google Scholar]
- Bakshi M, Singh S. Development of validated stability-indicating assay methods – critical review. J Pharm Biomed Anal. 2002;28:1011–1040. doi: 10.1016/s0731-7085(02)00047-x. [DOI] [PubMed] [Google Scholar]
- Lindahl TL, Baghaei F, Blixter IF, Gustafsson KM, Stigendal L, Sten-Linder M, Strandberg K, Hillarp A Expert Group on Coagulation of the External Quality Assurance in Laboratory Medicine in S. Effects of the oral, direct thrombin inhibitor dabigatran on five common coagulation assays. Thromb Haemost. 2011;105:371–378. doi: 10.1160/TH10-06-0342. [DOI] [PubMed] [Google Scholar]
- Stangier J, Wetzel K, Wienen W, Van Ryn J, Rathgen K. Measurement of the pharmacodynamic effect of dabigatran etexilate: thrombin clotting time. Thromb Haemost. 2009;7(Suppl. 2):674. [Google Scholar]
- Hawes EM, Deal AM, Funk-Adcock D, Gosselin R, Jeanneret C, Cook AM, Taylor JM, Whinna HC, Winkler AM, Moll S. Performance of coagulation tests in patients on therapeutic doses of dabigatran: a cross-sectional pharmacodynamic study based on peak and trough plasma levels. J Thromb Haemost. 2013;11:1493–1502. doi: 10.1111/jth.12308. [DOI] [PubMed] [Google Scholar]
- Antovic JP, Skeppholm M, Eintrei J, Boija EE, Soderblom L, Norberg EM, Onelov L, Ronquist-Nii Y, Pohanka A, Beck O, Hjemdahl P, Malmstrom RE. Evaluation of coagulation assays versus LC-MS/MS for determinations of dabigatran concentrations in plasma. Eur J Clin Pharmacol. 2013;69:1875–1881. doi: 10.1007/s00228-013-1550-4. [DOI] [PubMed] [Google Scholar]
- Avecilla ST, Ferrell C, Chandler WL, Reyes M. Plasma-diluted thrombin time to measure dabigatran concentrations during dabigatran etexilate therapy. Am J Clin Pathol. 2012;137:572–574. doi: 10.1309/AJCPAU7OQM0SRPZQ. [DOI] [PubMed] [Google Scholar]
- Douxfils J, Mullier F, Robert S, Chatelain C, Chatelain B, Dogne JM. Impact of dabigatran on a large panel of routine or specific coagulation assays. Laboratory recommendations for monitoring of dabigatran etexilate. Thromb Haemost. 2012;107:985–997. doi: 10.1160/TH11-11-0804. [DOI] [PubMed] [Google Scholar]
- Stangier J, Feuring M. Using the HEMOCLOT direct thrombin inhibitor assay to determine plasma concentrations of dabigatran. Blood Coagul Fibrinolysis. 2012;23:138–143. doi: 10.1097/MBC.0b013e32834f1b0c. [DOI] [PubMed] [Google Scholar]
- Douxfils J, Dogne JM, Mullier F, Chatelain B, Ronquist-Nii Y, Malmstrom RE, Hjemdahl P. Comparison of calibrated dilute thrombin time and aPTT tests with LC-MS/MS for the therapeutic monitoring of patients treated with dabigatran etexilate. Thromb Haemost. 2013;110:543–549. doi: 10.1160/TH13-03-0202. [DOI] [PubMed] [Google Scholar]
- Hapgood G, Butler J, Malan E, Chunilal S, Tran H. The effect of dabigatran on the activated partial thromboplastin time and thrombin time as determined by the Hemoclot thrombin inhibitor assay in patient plasma samples. Thromb Haemost. 2013;110:308–315. doi: 10.1160/TH13-04-0301. [DOI] [PubMed] [Google Scholar]
- Chin PK, Vella-Brincat JW, Barclay ML, Begg EJ. Perspective on dabigatran etexilate dosing: why not follow standard pharmacological principles? Br J Clin Pharmacol. 2012;74:734–740. doi: 10.1111/j.1365-2125.2012.04266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin PKL, Patterson DM, Zhang M, Jensen BP, Wright DFB, Barclay ML, Begg EJ. Coagulation assays and plasma fibrinogen concentrations in real-world patients with atrial fibrillation treated with dabigatran. Br J Clin Pharmacol. 2014;78:630–638. doi: 10.1111/bcp.12366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love JE, Ferrell C, Chandler WL. Monitoring direct thrombin inhibitors with a plasma diluted thrombin time. Thromb Haemost. 2007;98:234–242. [PubMed] [Google Scholar]
- Duffull SB, Wright DF, Al-Sallami HS, Zufferey PJ, Faed JM. Dabigatran: rational dose individualisation and monitoring guidance is needed. N Z Med J. 2012;125:148–154. [PubMed] [Google Scholar]
- Gong IY, Kim RB. Importance of pharmacokinetic profile and variability as determinants of dose and response to dabigatran, rivaroxaban, and apixaban. Can J Cardiol. 2013;29:S24–33. doi: 10.1016/j.cjca.2013.04.002. [DOI] [PubMed] [Google Scholar]
- Brunetti L, Bandali F. Dabigatran: is there a role for coagulation assays in guiding therapy? Ann Pharmacother. 2013;47:828–840. doi: 10.1345/aph.1R720. [DOI] [PubMed] [Google Scholar]
- Chin PK, Vella-Brincat JW, Walker SL, Barclay ML, Begg EJ. Dosing of dabigatran etexilate in relation to renal function and drug interactions at a tertiary hospital. Intern Med J. 2013;43:778–783. doi: 10.1111/imj.12170. [DOI] [PubMed] [Google Scholar]
- Chin PK, Barclay ML, Begg EJ. Rifampicin and dabigatran etexilate: a place for laboratory coagulation monitoring. Br J Clin Pharmacol. 2013;75:554–555. doi: 10.1111/j.1365-2125.2012.04408.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson BI, Dahl OE, Buller HR, Hettiarachchi R, Rosencher N, Bravo ML, Ahnfelt L, Piovella F, Stangier J, Kalebo P, Reilly P. A new oral direct thrombin inhibitor, dabigatran etexilate, compared with enoxaparin for prevention of thromboembolic events following total hip or knee replacement: the BISTRO II randomized trial. J Thromb Haemost. 2005;3:103–111. doi: 10.1111/j.1538-7836.2004.01100.x. [DOI] [PubMed] [Google Scholar]
- Holford NH. Target concentration intervention: beyond Y2K. Br J Clin Pharmacol. 2001;52(Suppl. 1):55S–59. doi: 10.1046/j.1365-2125.2001.0520s1055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FDA. 2010. Briefing information for the September 20, 2010 meeting of the cardiovascular and renal drugs advisory committee.
- Schiele F, van Ryn J, Canada K, Newsome C, Sepulveda E, Park J, Nar H, Litzenburger T. A specific antidote for dabigatran: functional and structural characterization. Blood. 2013;121:3554–3562. doi: 10.1182/blood-2012-11-468207. [DOI] [PubMed] [Google Scholar]
- Ezekowitz MD, Connolly S, Parekh A, Reilly PA, Varrone J, Wang S, Oldgren J, Themeles E, Wallentin L, Yusuf S. Rationale and design of RE-LY: randomized evaluation of long-term anticoagulant therapy, warfarin, compared with dabigatran. Am Heart J. 2009;157:805–810. doi: 10.1016/j.ahj.2009.02.005. [DOI] [PubMed] [Google Scholar]
- Larsen TB, Rasmussen LH, Skjoth F, Due KM, Callreus T, Rosenzweig M, Lip GY. Efficacy and safety of dabigatran etexilate and warfarin in ‘real-world’ patients with atrial fibrillation: a prospective nationwide cohort study. J Am Coll Cardiol. 2013;61:2264–2273. doi: 10.1016/j.jacc.2013.03.020. [DOI] [PubMed] [Google Scholar]
- Stangier J. Clinical pharmacokinetics and pharmacodynamics of the oral direct thrombin inhibitor dabigatran etexilate. Clin Pharmacokinet. 2008;47:285–295. doi: 10.2165/00003088-200847050-00001. [DOI] [PubMed] [Google Scholar]
- Stollberger C, Finsterer J. Concerns about storage and application of dabigatran and rivaroxaban. Eur J Clin Pharmacol. 2013;69:739–740. doi: 10.1007/s00228-012-1366-7. [DOI] [PubMed] [Google Scholar]
- Brunet A, Hermabessiere S, Benain X. Monday, 29 August 2011. Eur Heart J. 2011;32:313–631. [Google Scholar]
- Medsafe. Pradaxa: New Zealand datasheet [online]. Available at http://www.medsafe.govt.nz/profs/Datasheet/p/Pradaxacap.pdf (last accessed 28 October 2013)


