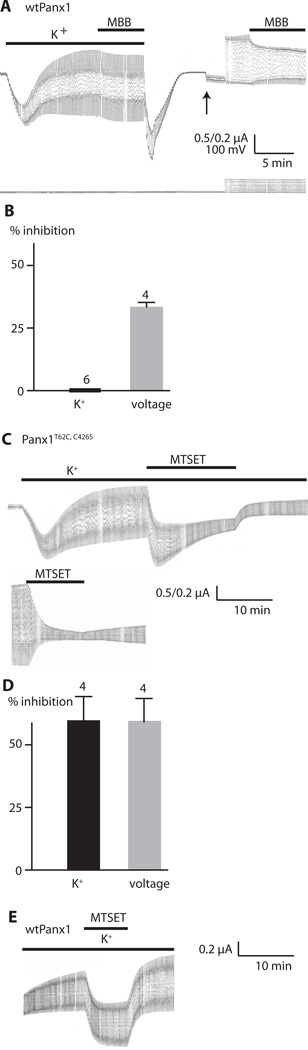
Fig. 4.
Modification by thiol reaction of Panx1 channels activated by K+ or by voltage. (A) Current traces recorded from an oocyte expressing wild-type Panx1 (wtPanx1). The membrane potential was held at −50 mV and 5 mV voltage pulses were applied at a rate of 0.1 Hz. Lower bar indicates KGlu (85 mM, K+); upper bar indicates presence of 100 µM MMB. KGlu and MMB were washed out and solution was replaced with oocyte Ringer solution, allowing, the membrane currents and conductance to return to baseline. After changing the gain (upward arrow), the pulse amplitude was increased to 100 mV (downward arrow) to stimulate voltage-activated Panx1 channel currents. Bar indicates the presence of 100 µM MBB. (B) Quantitative analysis of current inhibition by MBB for K+-activated and voltage-induced Panx1 channel currents. The number of measurements (N) is indicated above the bars. Data are shown as means ± SD. (C) Current traces from oocytes expressing Panx1T62C,C426S channels. Recording conditions were the same as in (A). Upper trace shows the effect of 1 mM MTSET on K+-activated Panx1 current. Lower trace shows the effect on voltage-activated current. Because of the reactivity of the thiol groups in K+ with MTSET, different oocytes were used for testing the effects on K+-activated and voltage-activated Panx1 currents. (D) Quantitative analysis of current inhibition by 1 mM MTSET for K+-activated and voltage-induced Panx1T62C,C426S channel currents. Data are shown as means ± SD. N is indicated above the bars. (E) Current traces of oocytes expressing wtPanx1. Recording conditions were the same as in (A). Panx1 was activated by K+ as indicated, and MTSET (1 mM) was applied as shown by the bar.