Figure 1.
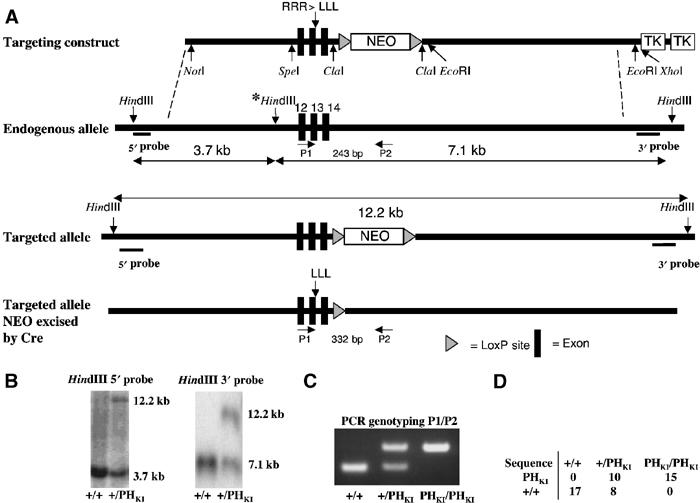
PDK1(PHKI/PHKI) knockin strategy. (A) Diagram depicting the knockin construct, the endogenous PDK1 allele containing exons 12–14, the targeted allele with the neomycin selection cassette still present, and the targeted allele with the neomycin cassette removed by Cre recombinase. The black boxes represent exons and the grey triangles represent LoxP sites. The three HindIII restriction sites present in the endogenous allele are shown, the middle site (marked with an asterisk), being removed upon targeting of the allele. (B) After positive and negative selection of cell lines (see Materials and methods), genomic DNA from the indicated cell lines was purified and digested overnight using HindIII, and the digested DNA was electrophoresed on a 1% agarose gel and transferred to nitrocellulose. The membrane was then incubated with a 32P-labelled 5′ or 3′ probe. In the case of the 5′ probe, the wild-type allele generates a 3.7 kb fragment whereas the knockin allele produces a 12.2 kb fragment. Similarly, the 3′ probe detects a fragment of 7.1 kb from the wild-type allele and 12.2 kb from the targeted knockin allele. (C) The indicated ES cell lines derived from E3 murine blastocysts (see Materials and methods) were genotyped using PCR primers P1 and P2. The wild-type allele generates a 243 bp product, while the knockin allele generates a 332 bp product due to the presence of the LoxP site and flanking region, which remains in an intronic region following Cre-mediated excision of the neomycin selection cassette. (D) Genomic DNA purified from the indicated ES cell lines was subjected to PCR to generate a product that encompasses the knockin mutation region. The resultant PCR products were ligated into the pCR-Topo 2.1 vector, transformed into Escherichia coli and clones sequenced. The numbers of the wild-type and knockin sequences obtained for each cell line are indicated.
