Figure 3.
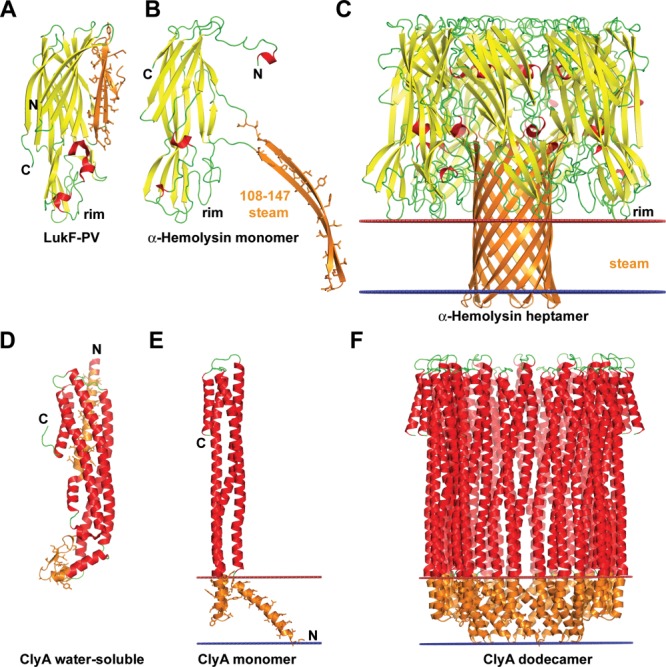
Conformational rearrangements of bacterial pore-forming toxins (PFT) upon binding to membranes. (A–C) Structures of β-PFT from Staphylococcus aureus: (A) soluble form of LukF monomer, a homologue of α-hemolysin (1PVL), (B) monomer of α-hemolysin from the oligomer (7AHL), (C) α-hemolysin heptamer (7AHL). Conformational changes in α-hemolysin include release of an amphiphilic β-hairpin (residues 108–147), oligomerization of seven molecules to create a stem domain with the central hydrophilic channel, and insertion of the stem domain into the membrane. (D–F) Structures of α-PFT from E. coli: (D) soluble form of ClyA monomer from E. coli (1QOY), (E) monomer of ClyA from the oligomer (2WCD), (F) ClyA dodecamer (2WCD). Conformational transitions of the ClyA toxin include detachment of β-tongue domain (residues 176–202) from the α-bundle, rearrangement of N-terminal α-helix, and formation of oligomers (octamers, dodecamers, or tridecamers) with circular pore inside the hydrophobic membrane core. The β-sheet is shown in yellow, the α-helices are shown in red, membrane-bound segments are shown in orange with side chains shown by sticks. Location of hydrophobic membrane boundaries are shown by lines: inner membrane leaflet is colored blue, outer leaflet is colored red.
