Figure 4.
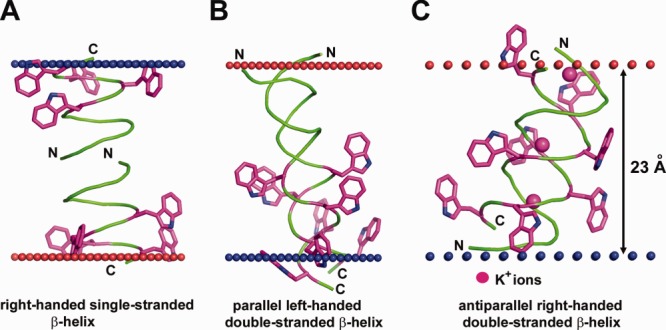
Different structures of antibiotic pentadecapeptide, gramicidin from Bacillus brevis. (A) Single-stranded right-handed β-helix of gramicidin A in the lipid bilayer (1MAG) forming a narrow ion channel with circular cross-section (diameter 4 Å). (B) Parallel left-handed double-stranded β-helix of gramicidin A in Ca+2–methanol solution (1MIC). (C) Antiparallel right-handed double-stranded β-helix of gramicidin D crystallized from methanol in complex with K+-ions (2IZQ). Insertion of gramicidin into the lipid bilayer decreases local hydrophobic thickness of the bilayer up to 20 Å and induces leakage of monovalent ions. Four Trp residues in each pentadecapeptide are shown by sticks colored purple (C-atoms) and blue (N-atoms). Calculated membrane boundaries from the OPM database are shown by red and blue spheres.
