Figure 5.
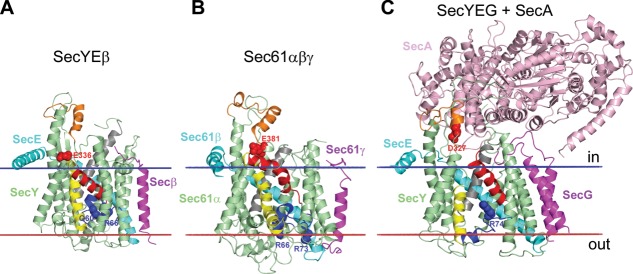
Protein translocation channels: (A) Archaebacterial SecYEβ from Methanococcus jannaschii (1RHZ); (B) canine Sec61αβγ (4CG5); (C) complex of SecYEG with SecA from E. coli (3DIN). The channel-forming Sec61α/SecY subunit has 10 TM α-helices arranged as two five-helix inverted repeats forming an hourglass-shaped channel between two repeats. The loop5–6 and loop8–9 (colored orange) contact with SecA or ribosome. The channel has a cytoplasmic funnel, the central hydrophobic ring, and external funnel filled by the TM2a helical plug (colored blue) with basic/polar residues (shown by blue spheres) that can electrostatically repel positive charges of translocated segments thus assisting in topology decision.322 Negatively charged residues from TM8 (shown by red spheres) also play topological role. In resting state (A, B), the lateral gate between TM2b (colored red) and TM7 (colored yellow) is closed, TM10 (colored gray) is moved outward, and the TM2a plug seals the pore ring, thus preventing ion leakage. In the SecYEG-SecA complex (C), insertion of two-helix finger of SecA into cytoplasmic funnel of SecY causes movement of the TM2a plug toward the periplasm and the displacement of the TM2b away from the TM7, thus creating a 5 Å-gap between these helices and providing partial opening of the translocation channel.92 In the open state, lateral gate between TM2b and TM7 is open and TM10 moves inward to the channel, which helps to release the translocated segment from channel into the lipid environment.94 Calculated membrane boundaries from the OPM database are shown by red and blue lines.
