Figure 11.
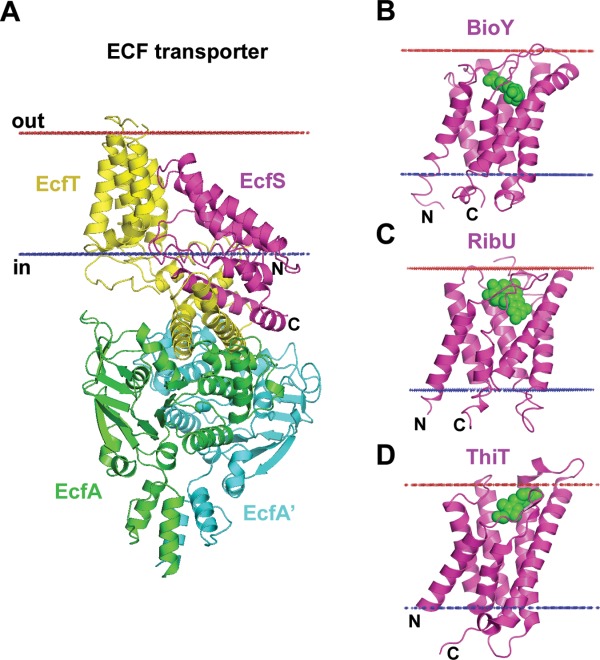
Reorientations of TM domains in energy coupling factor (ECF) transporters. (A) ECF transporter tetramer from Lactobacillus brevis (4HZU) is composed of TM energy-coupling component (EcfT) that links cytosolic ATPases, EcfA-EcfA', to the substrate-binding or S-protein (EcfS). In context of the ECF transporter tetramer, helices of the EcfS are highly tilted relative to the membrane plane (>45°) with the opening of the substrate-binding pocket approaching the cytoplasmic membrane interface. (B–D) Individual substrate-binding proteins are stable in membranes and are oriented along the membrane normal with substrate-binding pocket facing the extracellular space: (B) Biotin transporter S-protein, BioY, from Lactococcus lactis (4DVE); (C) Riboflavin transporter S-protein, RibU, from Staphylococcus aureus (3P5N); and (D) Thiamine transporter S-protein, ThiT, from Lactococcus lactis (3RLB). Substrates inside the binding pockets of S-proteins are shown by green spheres. Calculated hydrophobic membrane boundaries from the OPM database are shown by lines: blue at the cytoplasmic side and red at the extracellular side.
