In this study, normally immotile S2 cells are engineered to induce the formation of focal adhesions and cell motility by the transfection of a single gene encoding an integrin subunit. It is demonstrated that the focal adhesions recruit expected components and exhibit mechanosensitive behavior on integrin-ligand substrates of different stiffnesses.
Abstract
Focal adhesions are dynamic structures that interact with the extracellular matrix on the cell exterior and actin filaments on the cell interior, enabling cells to adhere and crawl along surfaces. We describe a system for inducing the formation of focal adhesions in normally non–ECM-adherent, nonmotile Drosophila S2 cells. These focal adhesions contain the expected molecular markers such as talin, vinculin, and p130Cas, and they require talin for their formation. The S2 cells with induced focal adhesions also display a nonpolarized form of motility on vitronectin-coated substrates. Consistent with findings in mammalian cells, the degree of motility can be tuned by changing the stiffness of the substrate and was increased after the depletion of PAK3, a p21-activated kinase. A subset of nonmotile, nonpolarized cells also exhibited focal adhesions that rapidly assembled and disassembled around the cell perimeter. Such cooperative and dynamic fluctuations of focal adhesions were decreased by RNA interference (RNAi) depletion of myosin II and focal adhesion kinase, suggesting that this behavior requires force and focal adhesion maturation. These results demonstrate that S2 cells, a cell line that is well studied for cytoskeletal dynamics and readily amenable to protein manipulation by RNAi, can be used to study the assembly and dynamics of focal adhesions and mechanosensitive cell motility.
INTRODUCTION
Cell motility is essential for the precise spatial and temporal organization of tissue morphogenesis, which gives rise to the elaborate, three-dimensional architecture of an organism (Friedl and Wolf, 2010). Cellular migration remains crucial throughout the lifetime of higher organisms, enabling processes such as wound healing and chemotactic responses in the immune system (Ridley et al., 2003). Metastasis demonstrates another manifestation of cell motility, in which transformed cells relocate from a primary tumor and colonize a secondary site (Thiery, 2002).
Different types of cells migrate by distinct mechanisms. Some amoeboid cells do not adhere or pull on the substrate but rather propulse themselves by blebbing the leading edge and contracting the rear. Other cells adhere tightly to substrates and use this traction to pull themselves along surfaces. Substrate adherence is often mediated by focal adhesions (FA), large protein complexes at the cell membrane that sense external stimuli and form mechanosensitive links between the extracellular matrix (ECM) and the actin cytoskeleton (Friedl and Wolf, 2010). Integrins are well-characterized transmembrane receptors that mediate these transmembrane connections. Integrins are αβ heterodimers with distinct extracellular domains that recognize diverse matrix ligands and a short cytoplasmic tail that binds to several actin-binding proteins (Huttenlocher and Horwitz, 2011). Integrins lack enzymatic activity, and their signaling requires the recruitment and activation of a complex network of more than 100 proteins that form the FA (Hynes, 2002), which include kinases (e.g., focal adhesion kinase [FAK] and adapter proteins [e.g., talin and pCas130]). The continuous formation and disassembly of adhesions is highly regulated, both spatially and temporally (Webb et al., 2004).
The fruit fly Drosophila melanogaster has proved to be a valuable model organism for the study of integrins, in part because flies contain fewer integrin subunits (5 α subunits [αPS1–5] and 2 β subunits [βPS and βν]) compared with mammals (18 α and 8 β subunits) (Hynes, 2002). Integrins function in a number of events in Drosophila development (Brown, 1993), and many different cell types in adult Drosophila, including hemocytes, require integrins for their migratory behavior (Narasimha and Brown, 2014; Siekhaus et al., 2010). However, thus far, a system to study integrin-dependent motility and focal adhesion function using Drosophila cells in culture has not been established.
More than a decade ago, it was shown that the expression of α-integrin chain in Drosophila S2 cells leads to the formation of an α,β-integrin complex that localizes to the cell surface and can produce cell adhesion to ECM (Bunch and Brower, 1992; Gotwals et al., 1994). In this paper, we show that α-integrin expression in Drosophila S2 induces the formation of functional, mechanosensitive FA when these cells are adhered to vitronectin. We also show that these S2 cells exhibit highly dynamic focal adhesion behavior and random cell crawling, which is not observed for normal S2 cells. We show that focal adhesion dynamics are dependent upon nonmuscle myosin II. We have also used RNA interference (RNAi) to dissect the roles of talin, FAK, and p21-activating kinase (Pak3) in focal adhesion formation and cell motility. This engineered cell line system provides a means of studying how FA form and affect the motile behavior of Drosophila cells.
RESULTS
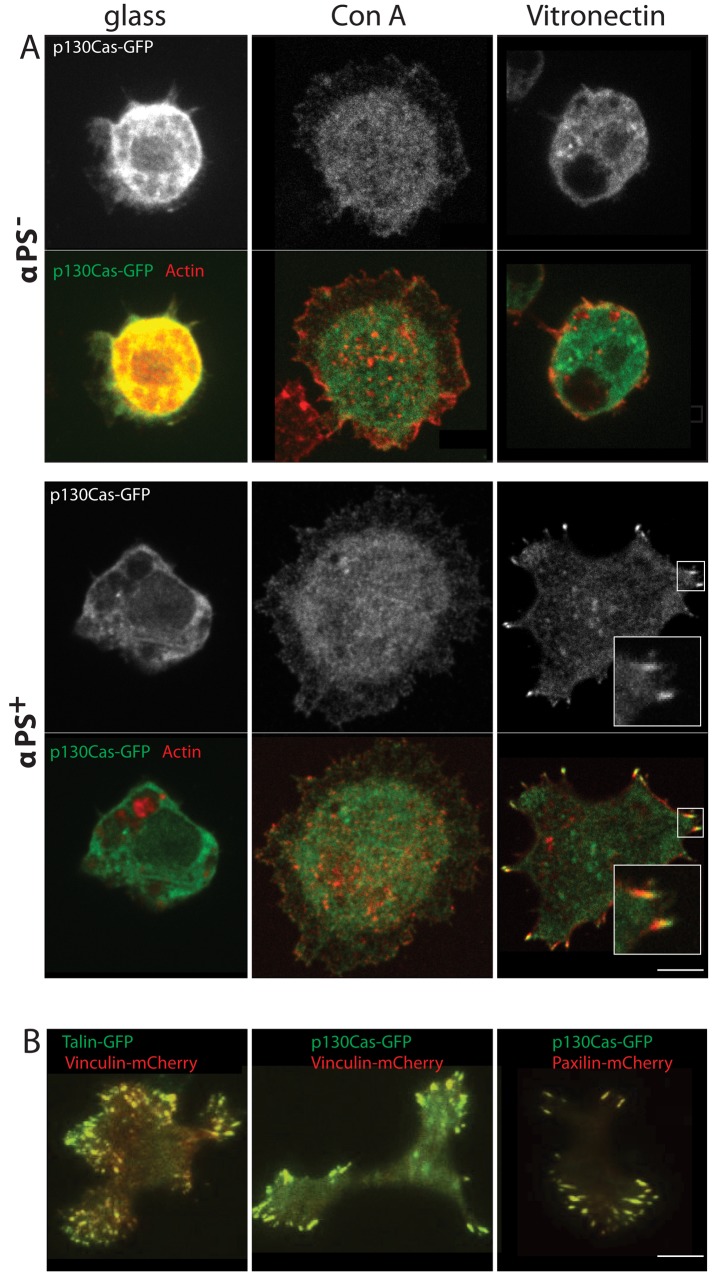
Drosophila Schneider 2U (S2U) cells are derived from the hemocytes that normally grow as round, nonadherent, and nonmotile cells. When plated on glass coverslips coated with the lectin concanavalin A (ConA), S2 cells flatten and spread to adopt a discoid morphology of approximately double their normal diameter but show no polarization or motility (Rogers et al., 2003; Figure 1A). S2 cells express very little endogenous α-integrin (Bunch and Brower, 1992; Gotwals et al., 1994). Previously it was shown that expression of the α-chain of integrin (αPS2m8) in S2 cells led to the expression of both α- and β-chains and the formation of functional integrin receptors on the cell surface, as evidenced by the ability of these cells to spread on ECM (Bunch and Brower, 1992; Gotwals et al., 1994).
FIGURE 1:
Expression of α-integrin in Drosophila S2 cells induces the formation of FA. (A) Drosophila S2 cells stably expressing the focal adhesion marker p130Cas-GFP were either induced (αPS+) or not induced (αPS−) for α-integrin expression and then plated on glass, ConA, or vitronectin for 2 h (see Materials and Methods). Cells were then fixed (6.4% formaldehyde) and incubated with Alexa Fluor 548–phalloidin for 1 h. The insets show a higher magnified view of the FA. Scale bar: 5 μm. (B) S2 cells expressing the indicated combinations of fluorescent protein–tagged versions of vinculin, talin, and paxilin. The yellow punctae reveal the superimposition of the GFP- and mCherry-tagged focal adhesion proteins. Scale bar: 5 μm.
We also found that S2 cells stably expressing the α-chain of integrin (αPS2+ cells) would avidly attach to and spread on surfaces coated with the ECM protein vitronectin. To better understand this process of cell adhesion, we next expressed the focal adhesion p130Cas–protein green fluorescent protein (p130Cas-GFP) in these αPS+ cells (Figure 1A). Normal αPS− cells did not localize p130Cas-GFP in any punctate structure at the cortex. Similarly, αPS+ cells plated on either glass or ConA did not localize p130CAS-GFP in surface punctae. However, when αPS+ cells were plated on vitronectin, they exhibited numerous surface punctae of p130Cas-GFP (Figure 1A). Bundles of actin filaments were seen emerging from these punctae, as is typical for FA (Figure 1A). However, these actin filaments were relatively short and did not extend into elongated stress fibers that extend through the cell, as seen in some cell types such as fibroblasts. Immunofluorescence staining also did not reveal an alternating, banded organization of α-actinin and nonmuscle myosin II (not shown), as is often found in stress fibers (Langanger et al., 1986).
To assess whether p130Cas clusters were indeed focal adhesion complexes, we coexpressed other well-characterized focal adhesion proteins, talin, vinculin, and paxilin, either with a GFP or mCherry tag. We verified that these fluorescently tagged proteins all colocalized in the same punctate structures that form when αPS+ cells are plated on vitronectin (Figure 1B). These results demonstrate that the punctae that form when αPS+ cells adhere to vitronectin contain the same focal adhesion components described in other adherent cell types. Thus we conclude that S2 cells contain the machinery for focal adhesion formation and that expression of the α-integrin chain might be sufficient to drive the formation of FA in otherwise poorly adherent S2 cells.
Migratory behavior and mechanosensing properties of integrin-expressing S2 cells
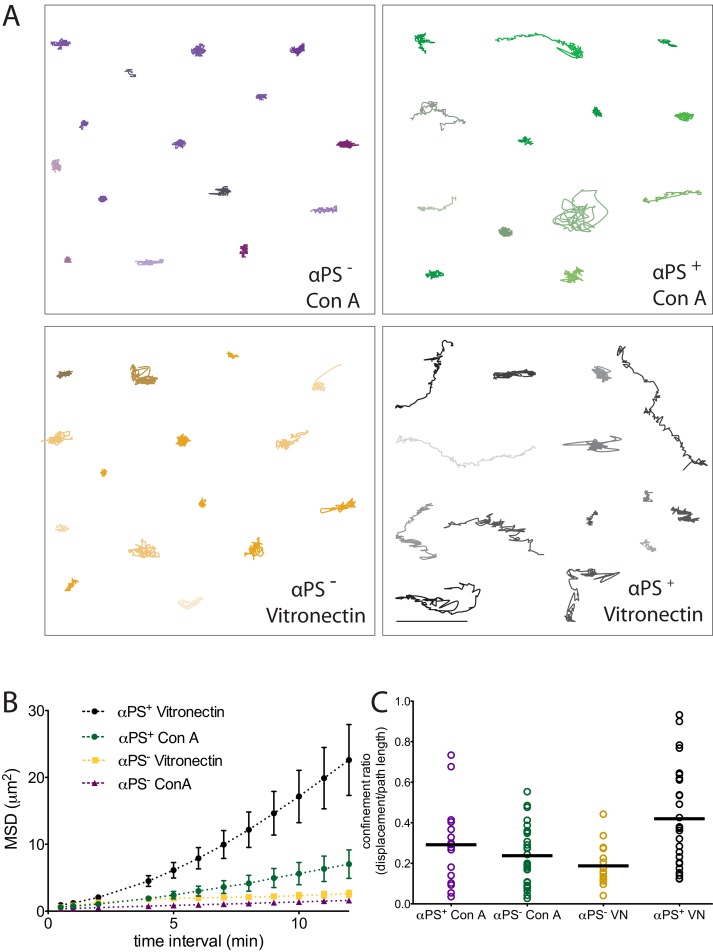
Next we wished to determine whether αPS2+ cells were capable of motility. We performed time-lapse imaging of αPS2+ and αPS2− cells expressing p130Cas-GFP on either vitronectin or ConA surfaces over a period of 1 h (Figure 2). To segment cells and determine their centroid, we used QuimP software (Dormann et al., 2002; Bosgraaf et al., 2009). The cell boundary was determined using the background fluorescence from p130Cas-GFP (Figure 2A; for more details see Materials and Methods). We used cell centroid trajectories to quantitate two parameters of cell movement: the mean square displacement (MSD) as a function of different time intervals (Figure 2B) and confinement ratio, which is the ratio of the final displacement distance to the total length of the path that the cell has traveled (Figure 2C). MSD is commonly used to evaluate random movement processes, such as diffusion or the random walk–like behavior of individual cells (Stokes and Lauffenburger, 1991). In the case of cell motility, the resulting average MSD curve gives information on the area explored by cells for a series of time intervals and a linear function of the MSD plot suggests random motion (Dieterich et al., 2008; Suraneni et al., 2012). The confinement ratio, on the other hand, is a measure of the persistence of the movement in one direction. A confinement ratio of 1 reflects directed cell movement (Beltman et al., 2009). The αPS2− cells spread on ConA, as previously described (Rogers et al., 2003), and exhibited very little movement of their centroid over time (Figure 2A). Likewise, these cells exhibited little movement on vitronectin. Some of the αPS2+ cells on ConA showed movement of their centroids, which likely reflects the fact that these cells are not well attached to ConA. However, on vitronectin, many of the of the αPS2+ cells displayed considerable movement of their centroid positions. Quantitative analysis of these cells' motility parameters showed a greater slope of the MSD-versus-time plot compared with αPS2− cells or αPS2+ cells on ConA-treated surfaces (Figure 2B and Supplemental Figure 1 for plots of MSDs of individual cells). However, the confinement ratio values were low, suggesting primarily random versus persistence motion (Figure 2C). In summary, these results show that αPS2+ cells can move on vitronectin surfaces but do not move persistently in one direction. Interestingly, we note that Drosophila S2R+ cells, which express both α- and β-integrin, can spread on an ECM but do not display motility (Jani and Schock, 2007), unlike what we observe for S2 cells. We do not understand the difference in this behavior for these two cell lines, but apparently some key component for motility is lacking in the S2R+ line.
FIGURE 2:
Cells expressing FA exhibit enhanced motility when plated on vitronectin. (A) The centroids of Drosophila S2 cells, in which α-integrin was induced (α-PS+) or not induced (α-PS−), that were plated on either vitronectin or ConA, were tracked every 30 s for 1 h. Examples of cell trajectories over 1 h are shown. Scale bar: 20 μm. (B) MSD is plotted at various time intervals for the indicated experiment treatments. The bars represent SEM. (For plots of each condition with single-cell trajectories see Supplemental Figure 1.) (C) Confinement ratio (ratio of the displacement of a cell to the total length that the cell traveled for 1 h) of cell trajectories, mean ± SD (n > 20 cells from two independent experiments).
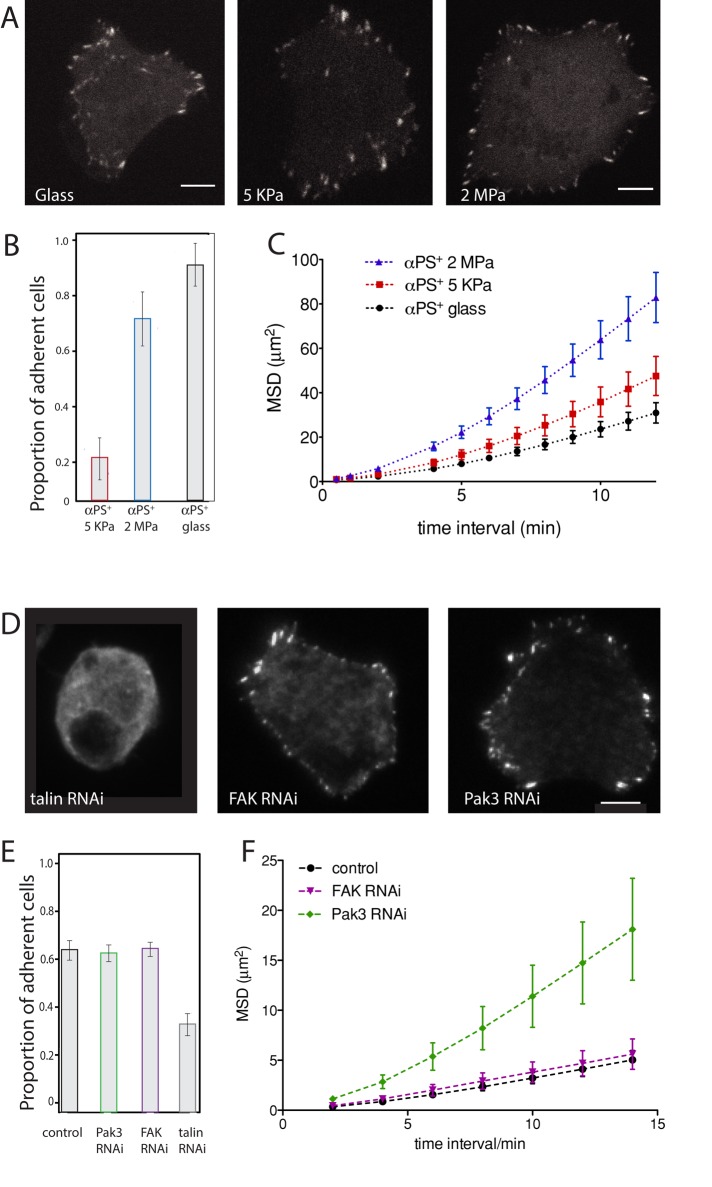
Mammalian cells with FA also have been shown to detect matrix rigidity via integrin-mediated adhesions and downstream mechanosensor protein signaling (Giannone and Sheetz, 2006). A soft matrix does not reinforce focal adhesion formation or cytoskeletal contractility and results in cell rounding (Ulrich et al., 2009). On the other hand, stiff substrates reinforce focal adhesion formation at cell protrusions (Peyton et al., 2008; Ulrich et al., 2009). Consequently, intermediate stiffness of the matrix has been shown to produce the fastest speeds of cell migration (Harland et al., 2011). We examined the behavior of αPS+ cells when placed on vitronectin-coated substrates of three different rigidities. We used the elastomeric system poly(dimethylsiloxane) (PDMS) cross-linked with a curing agent at two different ratios to produce surfaces with rigidities of 5 KPa (soft or compliant substrate) or 2 MPa (intermediate substrate) (Prager-Khoutorsky et al., 2011). A glass surface provided a third substrate of high rigidity (>20 MPa). FA (visualized with p130Cas–GFP) formed on both rigid and compliant substrates (Figure 3A). However, the percentage of cells that adhered to the soft (5 KPa) substrate was much lower when compared with intermediate and stiff substrates (Figure 3B). When the centroids of the cells were plotted over time, we found that the majority of cells were more mobile on the 2-MPa substrate than on the 5-kPa substrate. This was confirmed by quantitative analysis of the motion, which shows that intermediate (2-MPa) substrate displayed the steepest slope of the MSD plot (Figure 3C and Supplemental Figure 2 for plots of MSDs of individual cells). This increase in MSD was not accompanied by an increase in directionality, as confinement ratios on the different stiffness matrices were similar, signifying random cell migration (Supplemental Figure 3A). These results demonstrate that αPS2+ cells can detect the stiffness of a substrate and respond by modulating their motility.
FIGURE 3:
Response of FA to stiffness changes and effect of protein depletion on adhesion and motility. (A) Drosophila S2 cells expressing α-integrin (α-PS+) were plated on vitronectin-coated PDMS substrates of three different rigidities (glass, 5 KPa, or 2MPa). Scale bars: 5 μm). (B) The percentage of adherent cells was scored after a 1-h plating time, cell fixation, and Alexa Fluor 548–phalloidin staining. Two independent experiments were conducted, and in each experiment, 10 fields of view were imaged on a spinning-disk microscope (>100 cells counted per condition from two experiments). Bars indicates the confidence interval (of the binomial proportion) computed with the Pearson-Klopper method. (C) Cell movement was assayed on the different surfaces for 3 h. Graphs represent movement of the centroids of >30 cells from two experiments. (For plots of each condition with single-cell trajectories see Supplemental Figure 1.) (D) FA after talin, FAK, and Pak3 RNAi treatment. (E) Proportion of adherent vs. nonadherent cells after RNAi depletion of the talin, FAK, and Pak3. Cells were plated after 5 d of the RNAi treatment on a functionalized glass surface, images were acquired with a spinning-disk confocal microscope (>150 cells from two (talin RNAi) or three experiments). Bars indicate the 95% confidence interval (of the binomial proportion) computed with the Pearson-Klopper method. The t test is significantly different for talin-depleted cells compared with all other conditions (p < 0.05). (F) MSD of control and depleted cells, quantified using cell centroid trajectory over 3 h (>30 cells from three experiments).
We next examined the role of talin, FAK, and serine/threonine Pak3 in focal adhesion formation and random cell motility in αPS+ cells. Talin is a large cytoskeletal protein that binds β-integrin cytoplasmic tails (Geiger and Yamada, 2011) and activates integrins (Miranti and Brugge, 2002; Schlaepfer and Mitra, 2004). Drosophila has one talin gene, while vertebrates possess two. FAK tyrosine kinase elicits intracellular signal transduction pathways that promote the turnover of FA and facilitate cell migration (Miranti and Brugge, 2002; Schlaepfer and Mitra, 2004). In the Drosophila genome, there are three genes that encode Pak proteins: Pak1 and Pak3 belong to the Pak1 group, while Mbt belongs to the Pak2 group (Mentzel and Raabe, 2005; Baek et al., 2012). In contrast, there are six mammalian Pak genes: three genes in group 1 and three in group 2 (Hofmann et al., 2004). Pak1 family kinases control cell motility by regulating the organization and dynamic assembly of downstream effectors such as actin (Bokoch, 2003). We chose to study Pak3, because it has been shown to be involved in larvae wound closure, while perturbation of Pak1 did not affect cell motility (Baek et al., 2012). Furthermore, Pak3 RNAi depletion has been shown to induce S2 cells to exhibit a form of migratory behavior and enhanced lamellipodial dynamics, suggesting that Pak3 might be important for maintenance of a cell poised state (Asano et al., 2009).
After 5-d RNAi treatment of talin, cells formed few visible punctae of p130Cas-GFP (Figure 3D), suggesting that FA did not form. Furthermore, the adherence of talin-depleted cells to vitronectin-coated glass surfaces was decreased dramatically (Figure 3E). These results are largely consistent with findings from mammalian cells showing that talin is required for focal adhesion formation (Zhang et al., 2008).
In contrast to talin, after FAK RNAi, p130Cas-GFP containing FA still formed (Figure 3D), and the number of cells that adhered to vitronectin was similar to αPS2+ control cells (Figure 3E). Furthermore, long-term live-cell imaging, followed by MSD analysis, showed that cell motility parameters did not change after FAK depletion (Figure 3F). Pak3 RNAi also did not interfere with focal adhesion formation (Figure 3D) and did not affect the percentage of cells that adhered to vitronectin-coated glass surfaces (Figure 3E). Interestingly, long-term imaging of Pak3 RNAi cells showed an increase in the slope of the MSD plot (Figure 3F), indicating that the cells became more motile after depletion of this kinase. However, the movement was not directed, since there was no increase in the confinement ratio of these cells (Supplemental Figure 3B).
Collectively these results demonstrate that αPS2+ cells are mechanosensitive and can detect the stiffness of a substrate. Our RNAi results show that the assembly of de novo FAs in S2 cells requires talin, but neither FAK nor Pak3. FAK does not affect cell motility, while Pak3 increases the extent of random cell motility.
Behavior of FA in S2 cells
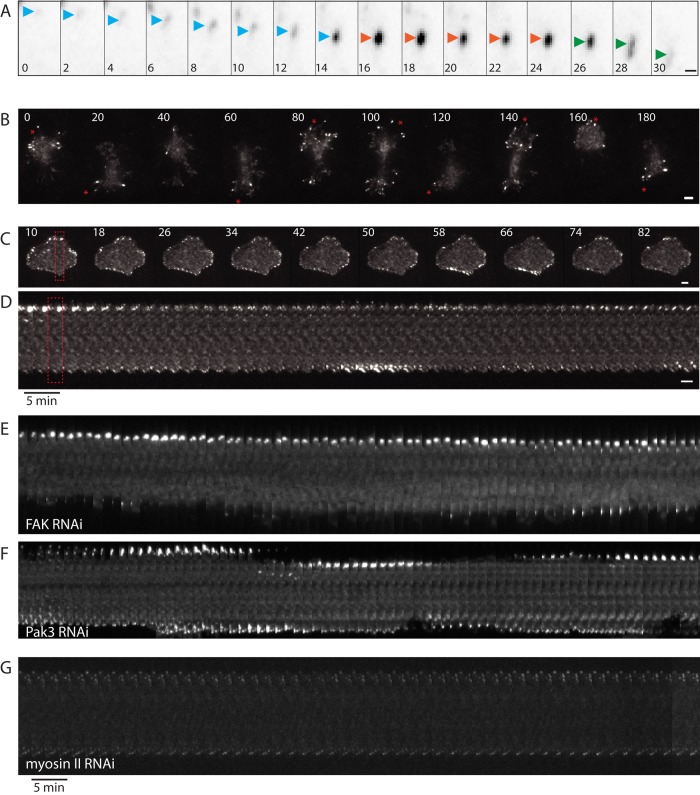
We next examined the dynamics of FA of αPS+ cells on vitronectin-coated glass surfaces for 3 h by time-lapse imaging using total internal reflection fluorescence (TIRF) microscopy. Most cells exhibited the well-characterized process of focal adhesion maturation and turnover (Webb et al., 2002; Choi et al., 2008), in which a nascent adhesion grew in its p130Cas–GFP intensity (assembly phase), maintained a constant intensity (stable phase), and then gradually lost intensity and disappeared (disassembly phase) (Figure 4A).
FIGURE 4:
Cells expressing integrin have different motility behaviors. Focal adhesion dynamic behavior requires FA maturation. (A) Example of a focal adhesion (labeled with p130Cas-GFP) turnover in α-PS+ cell plated on vitronectin: assembly (increasing intensity, blue arrowhead), maturation (constant intensity, red arrowhead), and disassembly (decreasing intensity, green arrowhead). Time: min; scale bar: 1 μm. (B and C) Still images from a time-lapse sequence of an α-PS+ cell showing motility (B) and an α-PS+ cell that is stationary (C) show clusters of FA appearing and disappearing in a synchronous manner at the perimeter (Supplemental Movie 1 and 2 correspond to the stills shown). Time: min; scale bar: 5 μm. (D) Kymograph of a cell slice (red dashed rectangle in C) shows the appearance and disappearance of FA at the bottom and top of the cell in an out-of-phase manner. (E–G) Kymograph of representative cell slices after FAK (E), Pak3 (F), and myosin II heavy-chain (G) depletion showing focal adhesion dynamics at opposite sides of a cell (see Figure 4D for more details). Scale bar: 5 μm.
We also compared the dynamics of FA in cells that exhibited motility (Figure 4B and Supplemental Movie 1) with ones that were stationary (Figure 4C and Supplemental Movie 2). Motile cells, which were 51 ± 20% of the population (mean ± SD, > 50 cells from four experiments), exhibited an irregular shape and were continually extending protrusions and changing shape. When a protrusion formed, the FA formed rapidly at the leading edge of the protrusion and then disappeared during the retraction of the protrusion (Figure 4A and Supplemental Movie 1). On the other hand, stationary cells tended to be more symmetric in their surface footprint. A subset of these stationary cells displayed FA that were largely static and nondynamic (19 ± 12% of the total cell population; mean ± SD, > 50 cells from four experiments). However, other stationary cells (30 ± 16%) exhibited very dynamic FA behavior; clusters of FAs appeared synchronously in a particular region of the cell perimeter and later synchronously disassembled (Figure 4C, Supplemental Movie 2, and Supplemental Figure 4A). This behavior is apparent in a kymograph, which shows the focal adhesion intensity increasing and decreasing in an out-of-phase manner at opposite sides of a cell (Figure 4D). Spectrum analysis did not reveal any defined periodicity of the appearance and disappearance of FA (unpublished data), suggesting that the cooperative assembly and disassembly of clusters of FA are largely stochastic in nature.
We next examined the contribution of FAK, Pak3, and nonmuscle myosin II in focal adhesion dynamics. The kymographs of FA at the cell membrane show that Pak3-depleted cells still had focal adhesion intensity increasing and decreasing at opposite sides of a cell (Figure 4F, Supplemental Movies 5 and 6, and Supplemental Figure 4C). In contrast, we found that the FAs were more stable in the FAK RNAi cells; these FA matured to large bright structures but tended to remain in place and not disassemble, as observed in a kymograph of a typical cell (Figure 4E, Supplemental Movies 3 and 4, and Supplemental Figure 4B). Nonmuscle myosin II was previously shown to be important for FA maturation in mammalian cells (Alexandrova et al., 2008; Choi et al., 2008). Drosophila has a single nonmuscle myosin II heavy chain, which we depleted by 5-d RNAi, a treatment previously shown to result in myosin depletion and a cytokinesis defect (Vale et al., 2009). Myosin II–depleted cells only exhibited small FAs (Supplemental Figure 5), which tended not to mature to larger structures. These FAs also were generally nondynamic and did not undergo cycles of maturation and disassembly in different regions of the cell periphery, as shown in the kymograph image of a cell slice (Figure 4F and Supplemental Movie 7). Taken together, these results show that factors known to be important for FA maturation and disassembly in mammalian cells (FAK and myosin II) are required for dynamic fluctuations of FA around the cell periphery of integrin-expressing S2 cells.
DISCUSSION
We developed a new system to induce the formation of FA in a controlled manner in S2 cells through the expression of an α-integrin chain. The formation of FA allows S2 cells to adhere to a vitronectin surface and also to sense and respond to the stiffness of the substrate, as described in mammalian cells. Thus this cell system provides the first opportunity to study focal adhesion formation and function in Drosophila cells in culture and should provide a useful tool to complement studies of these structures in intact flies and fly embryos (McMahon et al., 2010; Bulgakova et al., 2012; Pines et al., 2012).
Behavior of FA in S2 cells
The newly engineered S2 cell line has the ability to adhere to vitronectin-coated surfaces and show nondirected cell movement. Thus the expression of a single gene (α-integrin) is able to drive the formation of protein structures and signaling pathways that allow the cell to adhere and move. We also observed a cooperative assembly and disassembly of many FA on cells that do not move or show any overt polarization of their shape. This intriguing behavior suggests the existence of some dynamic signaling system that governs these spatial “waves” of focal adhesion assembly–disassembly, even in the absence of cell protrusion and retraction. This dynamic and cooperative focal adhesion behavior is displayed only by a subset of cells and shortly after plating on vitronectin (<3 h); after 24 h, virtually all cells exhibit nondynamic FA that change little in their intensity over an hour of observation (unpublished data). Case and Waterman (2011) also recently described waves of integrin and actin that travel along the ventral surface of adherent, migrating mammalian cells. These waves required a cycle of integrin assembly and disassembly engagement to the ECM. However, the waves are diffuse propagating bands of integrin and actin and are distinct from the FA, which do not exhibit this behavior in the same cells. Thus the cooperative, fluctuating focal adhesion behavior observed here appears to differ from these earlier described integrin waves. The nature of the intracellular signals that give rise to the fluctuating patterns of FA at the perimeter of αPS2+ S2 cells is presently not known. However, the process appears to be dependent on myosin II, which generates forces that are required for FA maturation. FAK also appears to influence the process of cooperative assembly and disassembly of FAs around the cell edge, since large, static FAs form around the cell perimeter after FAK depletion.
Functional and molecular characterization of S2 FA
Drosophila S2 cells are very amenable to RNAi protein depletion, and we have used this approach to study the role of three genes involved in FA turnover and downstream signaling: talin, FAK, and Pak3. Talin depletion generates phenotypes similar to those observed for integrin deficiencies in various model organisms, including Drosophila (Monkley et al., 2000; Brown et al., 2002; Cram et al., 2003). Further manipulation of talin function in fibroblasts showed that it is not required for initial cell spreading but is necessary for sustained cell adhesion and FA maturation (Zhang et al., 2008; Kopp et al., 2010). In agreement with these previous studies, we find that talin is essential for focal adhesion formation and adhesion to vitronectin by αPS2+ S2 cells.
The order of recruitment of talin and Fak to FA is still under debate. Studies using mouse knockouts or mammalian cell lines cells showed that talin does not require FAK for its recruitment to integrins at focal adhesion sites (Chen et al., 1995; Zhang et al., 2008; Wang et al., 2011). However, another recent study using MEF cells showed that FAK is indeed required for talin recruitment to nascent adhesions (Lawson et al., 2012). Our results in integrin-expressing S2 cells show that FAK is not required for talin recruitment, since we observe normal focal adhesion formation after FAK RNAi.
FAK depletion does not change cell motility, while Pak3-depleted cells have increased random cell motility. These results are largely consistent with previous results from mammalian cells. FAK is involved in turnover of FAs (Ilic et al., 1995; Mitra et al., 2005). Previous findings showed that FAK depletion results in a reduced rate of FA turnover and consequently to an increase in the level of steady-state FAs (Ilic et al., 1995; Webb et al., 2004). In our system, we show that FAK does not play a role in random cell migration and, as reported in the literature, it increases the level of static FAs at the cell edge.
When we depleted Pak3 in αPS2+ S2 cells, we observed a significant increase in nonpolarized cell motility and no alteration in FA dynamics around the cell edge in stationary cells. Pak3 depletion also has been linked to enhanced motility in nonadherent Drosophila cells (Asano et al., 2009). In Drosophila larvae, a deficiency in Pak3 results in poor epidermal wound healing (Baek et al., 2012), consistent with our results, which show an abnormality in cell motility. In vertebrates, Pak1 and Pak2 depletion leads to decreased FA turnover and an accompanying reduction in epithelial cell migration (Delorme-Walker et al., 2011). However, the role of Pak3 in vertebrate cell motility is less clear (Kreis et al., 2008). Pak3 expression in vertebrates is restricted to neurons (Bagrodia et al., 1995; Manser et al., 1995), where loss of function is associated with X-linked nonsyndromic mental retardation.
Future directions
In this work, we extend the use of the S2 cell line to study cell adhesion and focal adhesion formation. While αPS2+ S2 cells are more motile than normal S2 cells, they do not display persistent and directional movement. However, it might be possible to engineer this system further by introducing signaling modules, potentially coupled to receptor-ligand triggers, that could polarize the actin cytoskeleton and lead to directional motion. Such an engineered system would enable a better understanding of symmetry breaking and how cell polarity can be maintained and coupled to focal adhesion dynamics.
S2 cells are derived from hemocytes, which have the ability of undergo both directed migration (Evans et al., 2003; Wood and Jacinto, 2007) and random cell motility (Comber et al., 2013) during embryonic and late larval stages in Drosophila. Interestingly, the αPS2 integrin subunit is required for the invasive movement and transmigration of tissue barriers of embryonic macrophages from the head region into the tail in the whole organism (Siekhaus et al., 2010). Thus the αPS2+ S2 cells may be useful for addressing questions related to this interesting hemocyte behavior and understanding the molecular switches that cause hemocytes to change their migratory program during development. By adding factors to αPS2+ S2 cells (e.g., hormones or particular types of ECM or an epithelial tissue barrier), one also might be able to identify external cues that guide hemocytes inside the whole organism.
MATERIALS AND METHODS
Cell culture and stable cell lines
Drosophila S2U cells were maintained in Schneider's medium (Life Technologies, Carlsbad, CA) supplemented with 10% heat-inactivated fetal bovine serum (FBS; Life Technologies), penicillin, streptomycin, and kanamycin (Goshima et al., 2007). Full-length PS2m8 was kindly provided by Thomas A. Bunch and cloned into pHS (Life Technologies). cDNAs of pCas130, paxilin, and vinculin were obtained by PCR from Drosophila S2 cell cDNA. Vectors were cloned into metallothionein promoter pMT-GFP (Life Technologies) or pMT-Cherry vectors, as described previously (Goshima et al., 2007), using the Gateway system (Invitrogen). For generation of stable cell lines, the vectors were cotransfected with blasticidin and hygromycin plasmids in S2U cells and selected for five passages (Millar et al., 1994). pMT-GFP and pMT-mCherry were imaged after induction of gene expression with 50 μM CuSO4 overnight. The PS2m8 is under a heat-shock promoter (pHS), and its induction was achieved by maintaining cells at 37°C for 40 min and then transferring them to the 25°C incubator for 1 h before imaging cells.
For RNAi treatment, 6 × 105 α-PS-S2 cells were treated with 1 μg RNA and incubated for 30 min in serum-free Schneider's medium; this was followed by a 5-d recovery in Schneider's medium supplemented with 20% heat-inactivated FBS (Goshima et al., 2007). After 5 d, cells were resuspended and transferred to vitronectin or ConA-coated glass-bottom plates (Dot Scientific, Burton, MI). Vitronectin treatment was carried out by coating the surface with 70 μl of 10 μg/ml of vitronectin (EMD Millipore, Germany) overnight at 4°C; this was followed by three washes with phosphate-buffered saline (PBS) and growth medium. ConA treatment was performed by drying 70 μl of a 0.05 mg/ml ConA solution onto the bottom of each well.
PDMS surface coating
PDMS substrates of varying rigidities were prepared using a Sylgard 184 silicone elastomer kit (Dow Corning, Midland, MI; Prager-Khoutorsky et al., 2011). The silicone elastomer component was mixed with the curing agent, degassed, and spin-coated at 2000 rpm for 2 min on glass-bottom Microwell dishes (MatTek, Ashland, MA). Subsequently, cross-linking of the elastomer was carried out at 70°C for 4 h. The elastomer-to-curing agent ratios of 10:1 and 75:1 corresponded to Young's moduli 2 MPa and 5 kPa, respectively (Prager-Khoutorsky et al., 2011). Dishes with a layer of PDMS were functionalized with 10 μg/ml of vitronectin overnight at 4°C, and cells were plated after the surface was washed two times with PBS.
Cell motility measurements and live-cell imaging
For time-lapse imaging, cells growing in suspension were plated on functionalized surfaces, and after α-PS induction, cells were left to adhere to the surface for 1–2 h. Once cells were adherent, plates were taken to a TIRF microscope (TE2000; Nikon, Japan), where they were imaged with a 60×/1.49 NA oil objective with an Andor EM-CCD camera. For lower-resolution, time-lapse imaging was conducted in a spinning-disk microscope with a 16×/0.5 NA objective and a EM-CCD camera (Hamamatsu) and μManager microscopy software (Stuurman et al., 2007). Linear-contrast adjustments were performed on all images.
Image analysis
Movies were registered using the StackReg ImageJ plug-in (Thevenaz et al., 1998). To quantify cell movement, we used low-resolution time-lapse images and QuimP software (Dormann et al., 2002; Bosgraaf et al., 2009) to segment single cells and to determine the x,y coordinates of the centroid of each cell trajectory, as well as the displacement and path length. The x,y coordinates were exported to Excel and mean square displacement was calculated using the following formula:
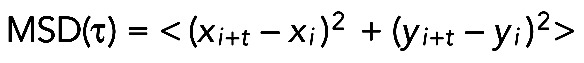 |
in which <> indicate averages, (xi, yi) are the coordinates of the cell at time i, and τ is the lag time between the two positions. After a series of MSD lag times were calculated, an ensemble average over several trajectories was plotted (Figures 2B and 3, C and F), as were individual traces for each cell trajectory (Supplemental Figures 1 and 2). Statistical analysis and data plotting was carried out on R for plots of Figure 3, B and E. All of the remaining plots were produced and analyzed in Prism (GraphPad).
For measurement of FA areas, live-cell images of control and zipper-depleted cells were loaded in Image J, and a threshold triangle was applied to select an FA. The area was measured, and the average area per frame was saved. The values were exported to GraphPad Prism, in which a histogram of the relative frequencies of average areas was constructed. Mean and SD were determined after fitting a curve to the data obtained.
Supplementary Material
Acknowledgments
We are grateful to Thomas A. Bunch for the pHS-PS2m8 vector. We thank F. Jay for helping with statistical analysis in R and C. Nyitray for help with PDMS coating. This work was supported by National Institutes of Health grant R01GM097312, and S.A.R. is supported by Human Frontier Science Program (HFSP) LT000745-2011-L.
Abbreviations used:
- ConA
concanavalin A
- ECM
extracellular matrix
- FA
focal adhesions
- FAK
focal adhesion kinase
- FBS
fetal bovine serum
- MSD
mean square displacement
- Pak3
p21-activating kinase
- PBS
phosphate-buffered saline
- PDMS
poly(dimethylsiloxane)
- RNAi
RNA interference
- TIRF
total internal reflection fluorescence.
Footnotes
*These authors contributed equally to this paper.
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E14-04-0863) on October 1, 2014.
REFERENCES
- Alexandrova AY, Arnold K, Schaub S, Vasiliev JM, Meister JJ, Bershadsky AD, Verkhovsky AB. Comparative dynamics of retrograde actin flow and focal adhesions: formation of nascent adhesions triggers transition from fast to slow flow. PLoS One. 2008;3:e3234. doi: 10.1371/journal.pone.0003234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asano Y, Jimenez-Dalmaroni A, Liverpool TB, Marchetti MC, Giomi L, Kiger A, Duke T, Baum B. Pak3 inhibits local actin filament formation to regulate global cell polarity. HFSP J. 2009;3:194–203. doi: 10.2976/1.3100548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baek SH, Cho HW, Kwon YC, Lee JH, Kim MJ, Lee H, Choe KM. Requirement for Pak3 in Rac1-induced organization of actin and myosin during Drosophila larval wound healing. FEBS Lett. 2012;586:772–777. doi: 10.1016/j.febslet.2012.01.061. [DOI] [PubMed] [Google Scholar]
- Bagrodia S, Taylor SJ, Creasy CL, Chernoff J, Cerione RA. Identification of a mouse p21Cdc42/Rac activated kinase. J Biol Chem. 1995;270:22731–22737. doi: 10.1074/jbc.270.39.22731. [DOI] [PubMed] [Google Scholar]
- Beltman JB, Maree AF, de Boer RJ. Analysing immune cell migration. Nat Rev Immunol. 2009;9:789–798. doi: 10.1038/nri2638. [DOI] [PubMed] [Google Scholar]
- Bokoch GM. Biology of the p21-activated kinases. Annu Rev Biochem. 2003;72:743–781. doi: 10.1146/annurev.biochem.72.121801.161742. [DOI] [PubMed] [Google Scholar]
- Bosgraaf L, van Haastert PJ, Bretschneider T. Analysis of cell movement by simultaneous quantification of local membrane displacement and fluorescent intensities using Quimp2. Cell Motil Cytoskeleton. 2009;66:156–165. doi: 10.1002/cm.20338. [DOI] [PubMed] [Google Scholar]
- Brown NH. Integrins hold Drosophila together. Bioessays. 1993;15:383–390. doi: 10.1002/bies.950150604. [DOI] [PubMed] [Google Scholar]
- Brown NH, Gregory SL, Rickoll WL, Fessler LI, Prout M, White RA, Fristrom JW. Talin is essential for integrin function in Drosophila. Dev Cell. 2002;3:569–579. doi: 10.1016/s1534-5807(02)00290-3. [DOI] [PubMed] [Google Scholar]
- Bulgakova NA, Klapholz B, Brown NH. Cell adhesion in Drosophila: versatility of cadherin and integrin complexes during development. Curr Opin Cell Biol. 2012;24:702–712. doi: 10.1016/j.ceb.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunch TA, Brower DL. Drosophila PS2 integrin mediates RGD-dependent cell-matrix interactions. Development. 1992;116:239–247. doi: 10.1242/dev.116.1.239. [DOI] [PubMed] [Google Scholar]
- Case LB, Waterman CM. Adhesive F-actin waves: a novel integrin-mediated adhesion complex coupled to ventral actin polymerization. PLoS One. 2011;6:e26631. doi: 10.1371/journal.pone.0026631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HC, Appeddu PA, Parsons JT, Hildebrand JD, Schaller MD, Guan JL. Interaction of focal adhesion kinase with cytoskeletal protein talin. J Biol Chem. 1995;270:16995–16999. doi: 10.1074/jbc.270.28.16995. [DOI] [PubMed] [Google Scholar]
- Choi CK, Vicente-Manzanares M, Zareno J, Whitmore LA, Mogilner A, Horwitz AR. Actin and alpha-actinin orchestrate the assembly and maturation of nascent adhesions in a myosin II motor-independent manner. Nat Cell Biol. 2008;10:1039–1050. doi: 10.1038/ncb1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comber K, Huelsmann S, Evans I, Sanchez-Sanchez BJ, Chalmers A, Reuter R, Wood W, Martin-Bermudo MD. A dual role for the βPS integrin myospheroid in mediating Drosophila embryonic macrophage migration. J Cell Sci. 2013;126:3475–3484. doi: 10.1242/jcs.129700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cram EJ, Clark SG, Schwarzbauer JE. Talin loss-of-function uncovers roles in cell contractility and migration in C. elegans. J Cell Sci. 2003;116:3871–3878. doi: 10.1242/jcs.00705. [DOI] [PubMed] [Google Scholar]
- Delorme-Walker VD, Peterson JR, Chernoff J, Waterman CM, Danuser G, DerMardirossian C, Bokoch GM. Pak1 regulates focal adhesion strength, myosin IIA distribution, and actin dynamics to optimize cell migration. J Cell Biol. 2011;193:1289–1303. doi: 10.1083/jcb.201010059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieterich P, Klages R, Preuss R, Schwab A. Anomalous dynamics of cell migration. Proc Natl Acad Sci USA. 2008;105:459–463. doi: 10.1073/pnas.0707603105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dormann D, Libotte T, Weijer CJ, Bretschneider T. Simultaneous quantification of cell motility and protein-membrane-association using active contours. Cell Motil Cytoskeleton. 2002;52:221–230. doi: 10.1002/cm.10048. [DOI] [PubMed] [Google Scholar]
- Evans CJ, Hartenstein V, Banerjee U. Thicker than blood: conserved mechanisms in Drosophila and vertebrate hematopoiesis. Dev Cell. 2003;5:673–690. doi: 10.1016/s1534-5807(03)00335-6. [DOI] [PubMed] [Google Scholar]
- Friedl P, Wolf K. Plasticity of cell migration: a multiscale tuning model. J Cell Biol. 2010;188:11–19. doi: 10.1083/jcb.200909003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger B, Yamada KM. Molecular architecture and function of matrix adhesions. Cold Spring Harb Perspect Biol. 2011;3:a005033. doi: 10.1101/cshperspect.a005033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannone G, Sheetz MP. Substrate rigidity and force define form through tyrosine phosphatase and kinase pathways. Trends Cell Biol. 2006;16:213–223. doi: 10.1016/j.tcb.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Goshima G, Wollman R, Goodwin SS, Zhang N, Scholey JM, Vale RD, Stuurman N. Genes required for mitotic spindle assembly in Drosophila S2 cells. Science. 2007;316:417–421. doi: 10.1126/science.1141314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotwals PJ, Fessler LI, Wehrli M, Hynes RO. Drosophila PS1 integrin is a laminin receptor and differs in ligand specificity from PS2. Proc Natl Acad Sci USA. 1994;91:11447–11451. doi: 10.1073/pnas.91.24.11447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harland B, Walcott S, Sun SX. Adhesion dynamics and durotaxis in migrating cells. Phys Biol. 2011;8:015011. doi: 10.1088/1478-3975/8/1/015011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann C, Shepelev M, Chernoff J. The genetics of Pak. J Cell Sci. 2004;117:4343–4354. doi: 10.1242/jcs.01392. [DOI] [PubMed] [Google Scholar]
- Huttenlocher A, Horwitz AR. Integrins in cell migration. Cold Spring Harb Perspect Biol. 2011;3:a005074. doi: 10.1101/cshperspect.a005074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- Ilic D, Furuta Y, Kanazawa S, Takeda N, Sobue K, Nakatsuji N, Nomura S, Fujimoto J, Okada M, Yamamoto T. Reduced cell motility and enhanced focal adhesion contact formation in cells from FAK-deficient mice. Nature. 1995;377:539–544. doi: 10.1038/377539a0. [DOI] [PubMed] [Google Scholar]
- Jani K, Schock F. Zasp is required for the assembly of functional integrin adhesion sites. J Cell Biol. 2007;179:1583–1597. doi: 10.1083/jcb.200707045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp PM, Bate N, Hansen TM, Brindle NP, Praekelt U, Debrand E, Coleman S, Mazzeo D, Goult BT, Gingras AR, et al. Studies on the morphology and spreading of human endothelial cells define key inter- and intramolecular interactions for talin1. Eur J Cell Biol. 2010;89:661–673. doi: 10.1016/j.ejcb.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreis P, Rousseau V, Thevenot E, Combeau G, Barnier JV. The four mammalian splice variants encoded by the p21-activated kinase 3 gene have different biological properties. J Neurochem. 2008;106:1184–1197. doi: 10.1111/j.1471-4159.2008.05474.x. [DOI] [PubMed] [Google Scholar]
- Langanger G, Moeremans M, Daneels G, Sobieszek A, De Brabander M, De Mey J. The molecular organization of myosin in stress fibers of cultured cells. J Cell Biol. 1986;102:200–209. doi: 10.1083/jcb.102.1.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson C, Lim ST, Uryu S, Chen XL, Calderwood DA, Schlaepfer DD. FAK promotes recruitment of talin to nascent adhesions to control cell motility. J Cell Biol. 2012;196:223–232. doi: 10.1083/jcb.201108078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manser E, Chong C, Zhao ZS, Leung T, Michael G, Hall C, Lim L. Molecular cloning of a new member of the p21-Cdc42/Rac-activated kinase (PAK) family. J Biol Chem. 1995;270:25070–25078. doi: 10.1074/jbc.270.42.25070. [DOI] [PubMed] [Google Scholar]
- McMahon A, Reeves GT, Supatto W, Stathopoulos A. Mesoderm migration in Drosophila is a multi-step process requiring FGF signaling and integrin activity. Development. 2010;137:2167–2175. doi: 10.1242/dev.051573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mentzel B, Raabe T. Phylogenetic and structural analysis of the Drosophila melanogaster p21-activated kinase DmPAK3. Gene. 2005;349:25–33. doi: 10.1016/j.gene.2004.12.030. [DOI] [PubMed] [Google Scholar]
- Millar NS, Buckingham SD, Sattelle DB. Stable expression of a functional homo-oligomeric Drosophila GABA receptor in a Drosophila cell line. Proc Biol Sci. 1994;258:307–314. doi: 10.1098/rspb.1994.0178. [DOI] [PubMed] [Google Scholar]
- Miranti CK, Brugge JS. Sensing the environment: a historical perspective on integrin signal transduction. Nat Cell Biol. 2002;4:E83–E90. doi: 10.1038/ncb0402-e83. [DOI] [PubMed] [Google Scholar]
- Mitra SK, Hanson DA, Schlaepfer DD. Focal adhesion kinase: in command and control of cell motility. Nat Rev Mol Cell Biol. 2005;6:56–68. doi: 10.1038/nrm1549. [DOI] [PubMed] [Google Scholar]
- Monkley SJ, Zhou XH, Kinston SJ, Giblett SM, Hemmings L, Priddle H, Brown JE, Pritchard CA, Critchley DR, Fassler R. Disruption of the talin gene arrests mouse development at the gastrulation stage. Dev Dyn. 2000;219:560–574. doi: 10.1002/1097-0177(2000)9999:9999<::AID-DVDY1079>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Narasimha M, Brown NH. Integrins and associated proteins in Drosophila development. Madame Curie Bioscience Database [Internet], Austin, TX: Landes Bioscience. 2014. www.ncbi.nlm.nih.gov/books/NBK6575(accessed 2014)
- Peyton SR, Kim PD, Ghajar CM, Seliktar D, Putnam AJ. The effects of matrix stiffness and RhoA on the phenotypic plasticity of smooth muscle cells in a 3-D biosynthetic hydrogel system. Biomaterials. 2008;29:2597–2607. doi: 10.1016/j.biomaterials.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pines M, Das R, Ellis SJ, Morin A, Czerniecki S, Yuan L, Klose M, Coombs D, Tanentzapf G. Mechanical force regulates integrin turnover in Drosophila in vivo. Nat Cell Biol. 2012;14:935–943. doi: 10.1038/ncb2555. [DOI] [PubMed] [Google Scholar]
- Prager-Khoutorsky M, Lichtenstein A, Krishnan R, Rajendran K, Mayo A, Kam Z, Geiger B, Bershadsky AD. Fibroblast polarization is a matrix-rigidity-dependent process controlled by focal adhesion mechanosensing. Nat Cell Biol. 2011;13:1457–1465. doi: 10.1038/ncb2370. [DOI] [PubMed] [Google Scholar]
- Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, Borisy G, Parsons JT, Horwitz AR. Cell migration: integrating signals from front to back. Science. 2003;302:1704–1709. doi: 10.1126/science.1092053. [DOI] [PubMed] [Google Scholar]
- Rogers SL, Wiedemann U, Stuurman N, Vale RD. Molecular requirements for actin-based lamella formation in Drosophila S2 cells. J Cell Biol. 2003;162:1079–1088. doi: 10.1083/jcb.200303023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaepfer DD, Mitra SK. Multiple connections link FAK to cell motility and invasion. Curr Opin Genet Dev. 2004;14:92–101. doi: 10.1016/j.gde.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Siekhaus D, Haesemeyer M, Moffitt O, Lehmann R. RhoL controls invasion and Rap1 localization during immune cell transmigration in Drosophila. Nat Cell Biol. 2010;12:605–610. doi: 10.1038/ncb2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes CL, Lauffenburger DA. Analysis of the roles of microvessel endothelial cell random motility and chemotaxis in angiogenesis. J Theor Biol. 1991;152:377–403. doi: 10.1016/s0022-5193(05)80201-2. [DOI] [PubMed] [Google Scholar]
- Stuurman N, Amodaj N, Vale RD. Micro-Manager: open source software for light microscope images. Microsc Today. 2007;15:42–43. [Google Scholar]
- Suraneni P, Rubinstein B, Unruh JR, Durnin M, Hanein D, Li R. The Arp2/3 complex is required for lamellipodia extension and directional fibroblast cell migration. J Cell Biol. 2012;197:239–251. doi: 10.1083/jcb.201112113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thevenaz P, Ruttimann UE, Unser M. A pyramid approach to subpixel registration based on intensity. IEEE Trans Image Process. 1998;7:27–41. doi: 10.1109/83.650848. [DOI] [PubMed] [Google Scholar]
- Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2:442–454. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- Ulrich TA, de Juan Pardo EM, Kumar S. The mechanical rigidity of the extracellular matrix regulates the structure, motility, and proliferation of glioma cells. Cancer Res. 2009;69:4167–4174. doi: 10.1158/0008-5472.CAN-08-4859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vale RD, Spudich JA, Griffis ER. Dynamics of myosin, microtubules, and kinesin-6 at the cortex during cytokinesis in Drosophila S2 cells. J Cell Biol. 2009;186:727–738. doi: 10.1083/jcb.200902083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Ballestrem C, Streuli CH. The C terminus of talin links integrins to cell cycle progression. J Cell Biol. 2011;195:499–513. doi: 10.1083/jcb.201104128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb DJ, Donais K, Whitmore LA, Thomas SM, Turner CE, Parsons JT, Horwitz AF. FAK-Src signalling through paxillin, ERK and MLCK regulates adhesion disassembly. Nat Cell Biol. 2004;6:154–161. doi: 10.1038/ncb1094. [DOI] [PubMed] [Google Scholar]
- Webb DJ, Parsons JT, Horwitz AF. Adhesion assembly, disassembly and turnover in migrating cells—over and over and over again. Nat Cell Biol. 2002;4:E97–E100. doi: 10.1038/ncb0402-e97. [DOI] [PubMed] [Google Scholar]
- Wood W, Jacinto A. Drosophila melanogaster embryonic haemocytes: masters of multitasking. Nat Rev Mol Cell Biol. 2007;8:542–551. doi: 10.1038/nrm2202. [DOI] [PubMed] [Google Scholar]
- Zhang X, Jiang G, Cai Y, Monkley SJ, Critchley DR, Sheetz MP. Talin depletion reveals independence of initial cell spreading from integrin activation and traction. Nat Cell Biol. 2008;10:1062–1068. doi: 10.1038/ncb1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.