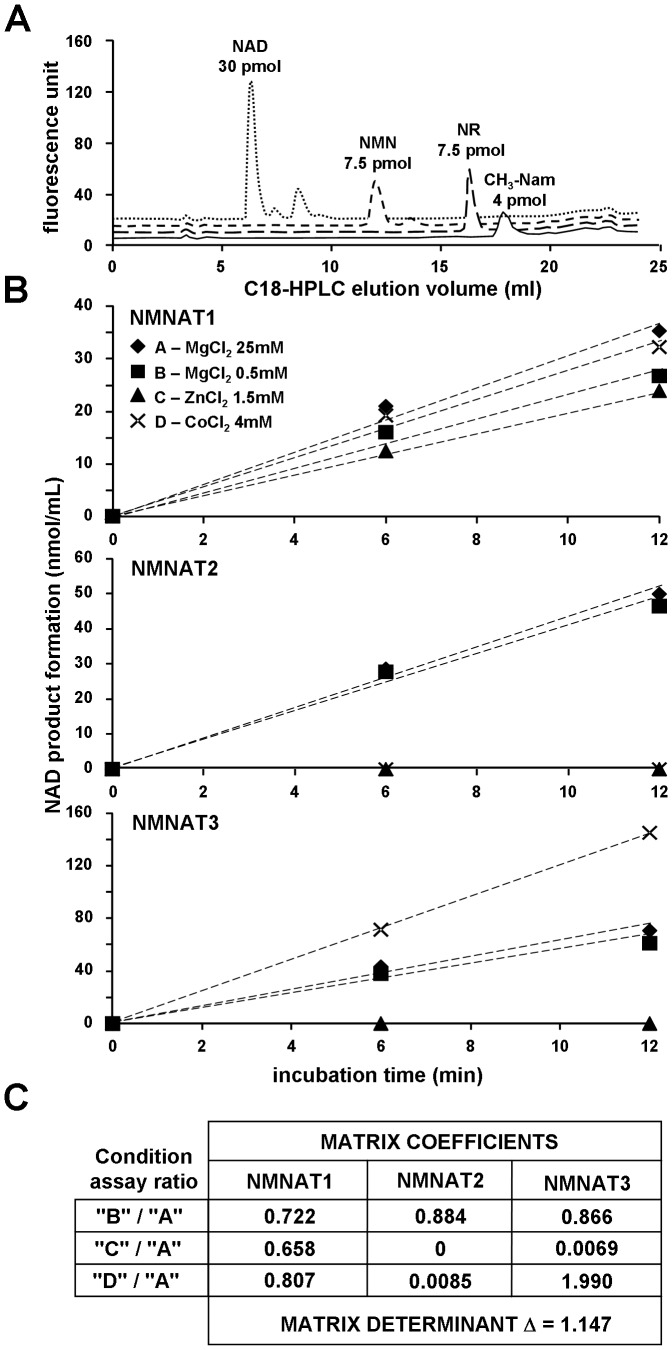
Figure 2. Spectrofluorometric analysis of pyridine metabolites, and discrimination assay of NMNAT isozymes.
A, C18-HPLC fluorescence profiles (excitation 380 nm, emission 440 nm) of pure standard pyridine metabolites after chemical derivatization with acetophenone. By this procedure, NMN, NAD, NR, and CH3-Nam are all converted into the respective highly fluorescent derivatives. B, discrimination assays of mouse NMNAT isozymes; C, calculation matrix. Each isozyme (pure recombinant) was assayed under four conditions exploiting its own metal ion dependence, i.e. in the presence of 25 mM MgCl2 (♦, condition A = arbitrary reference), or 0.5 mM MgCl2 (▪, condition B), or 1.5 mM ZnCl2 (▴, condition C), or 4 mM CoCl2 (×, condition D). The NAD product formation was evaluated by a NAD cycling assay (see Methods). The reaction rates shown were needed to generate the matrix coefficients used in this work to calculate the individual activities of the three mouse NMNAT isozymes in the tissue extracts. The matrix determinant in this example is 1.147 (absolute value) [18].