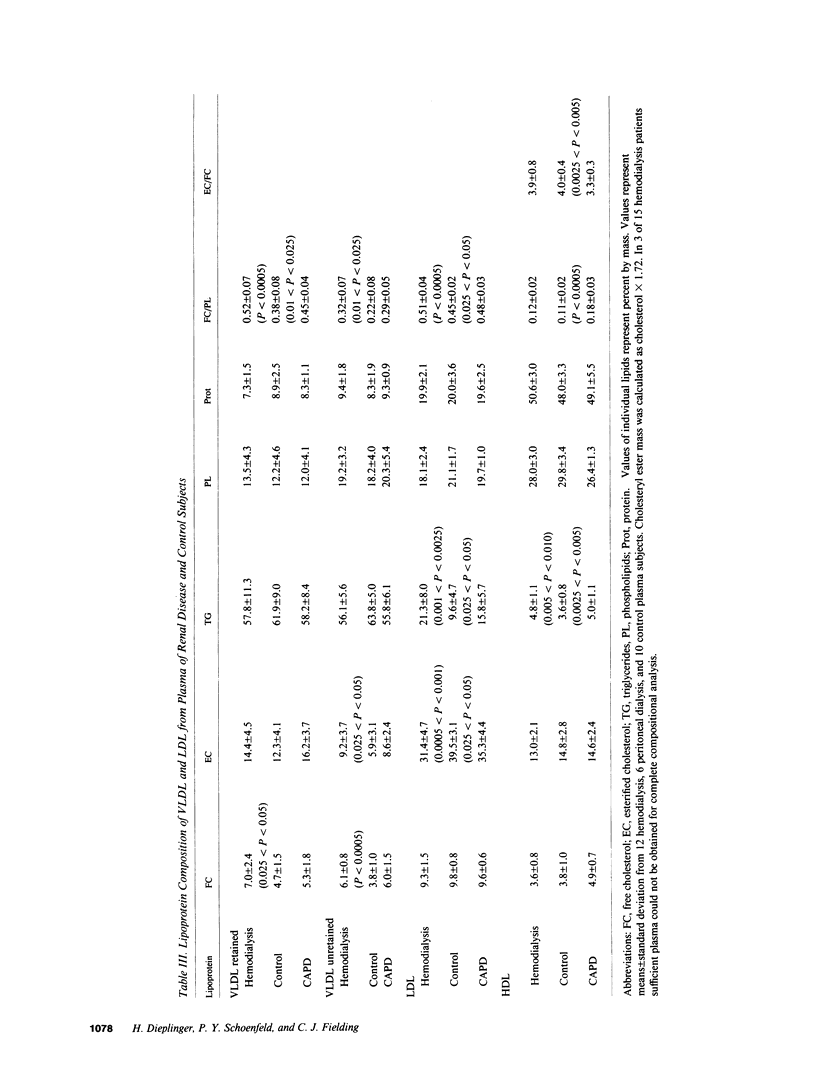
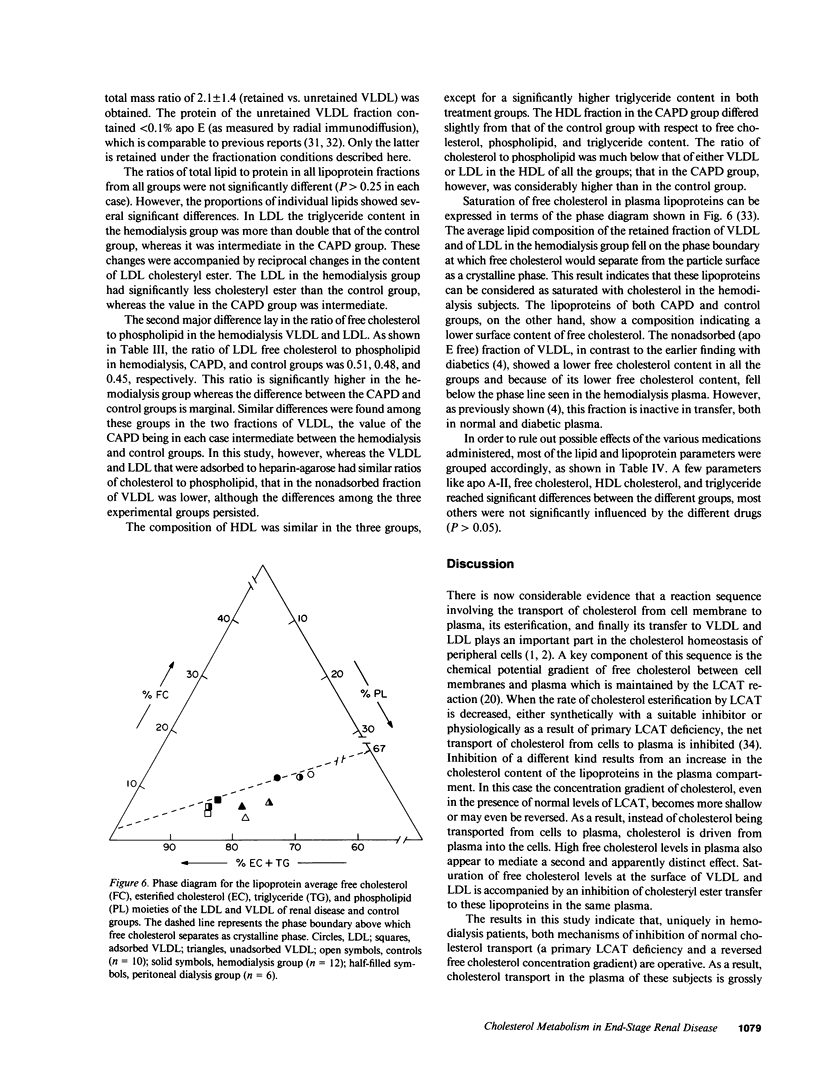
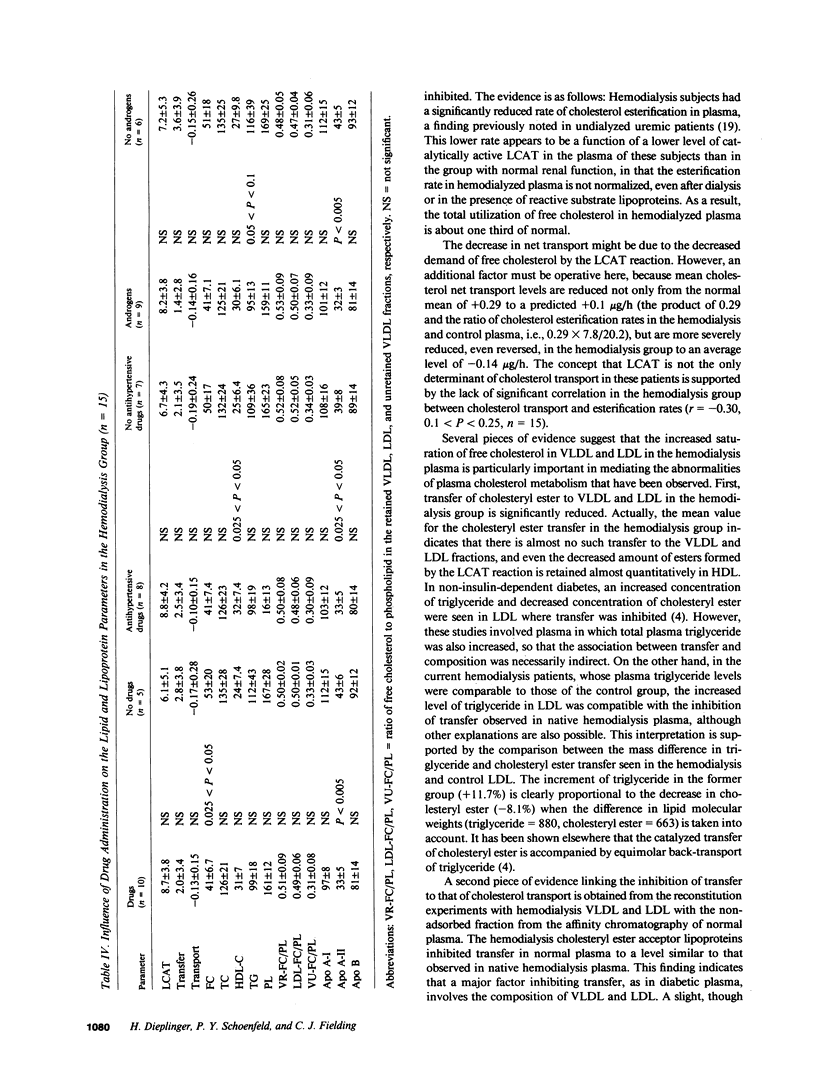
Abstract
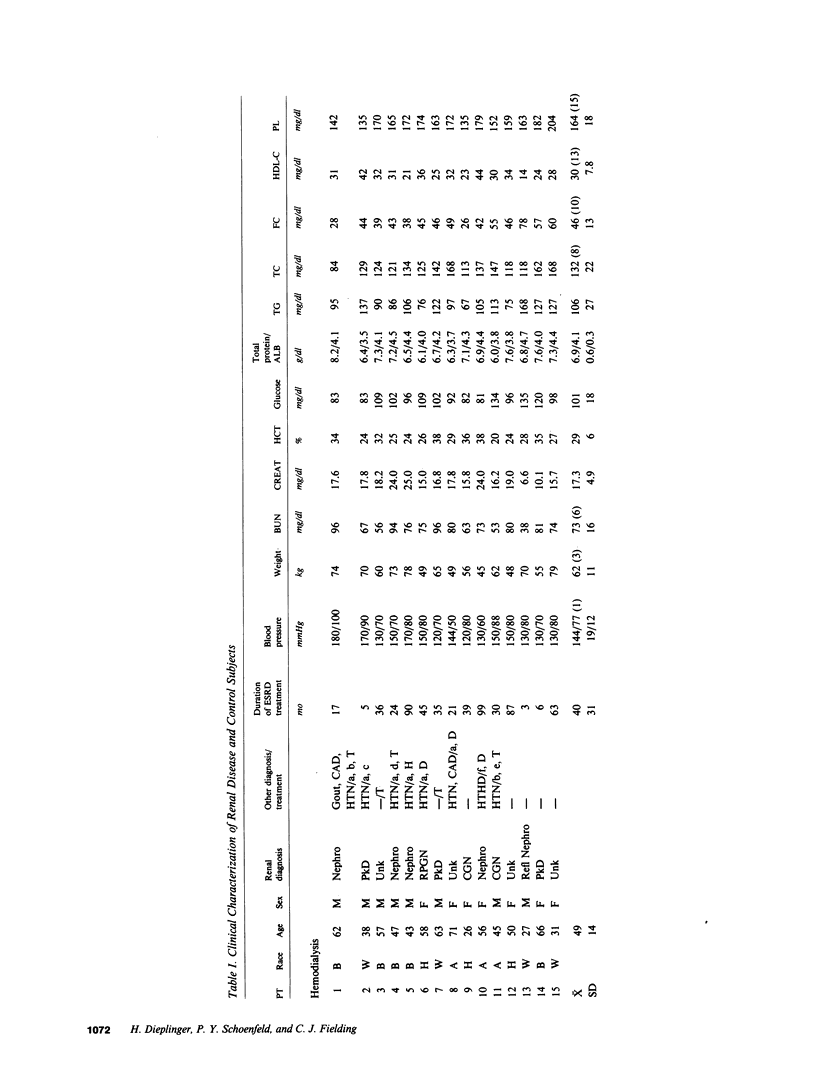
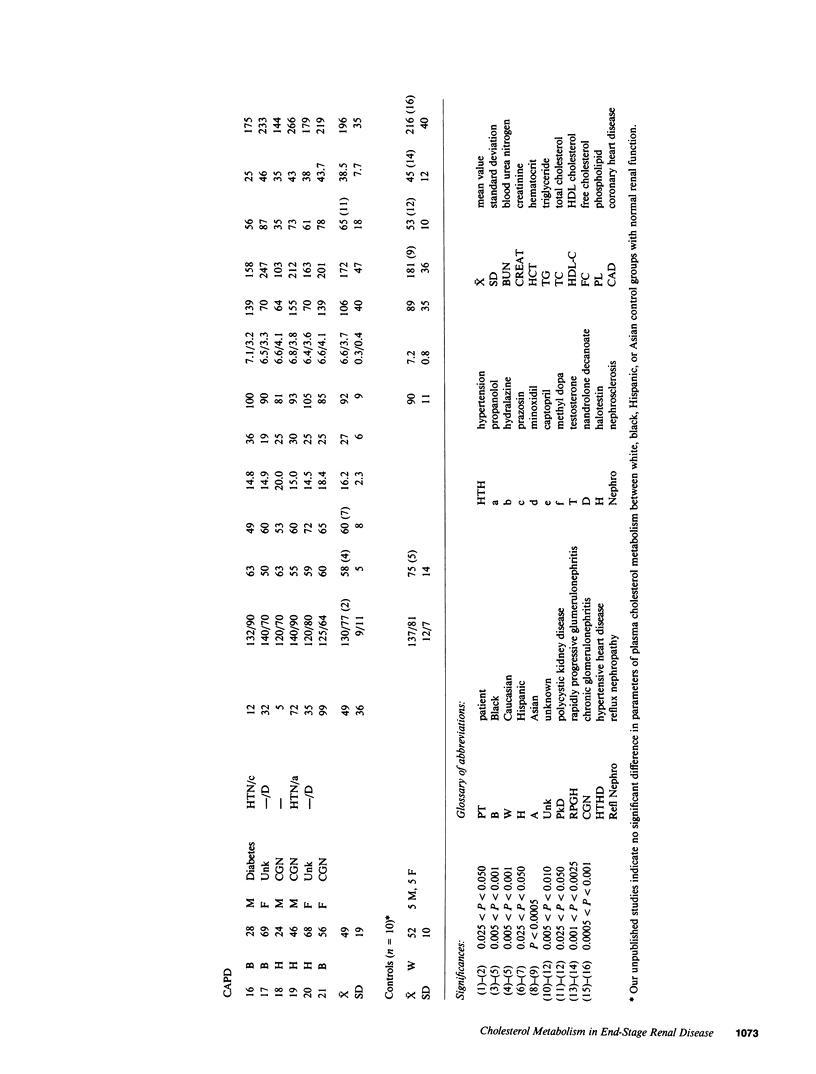
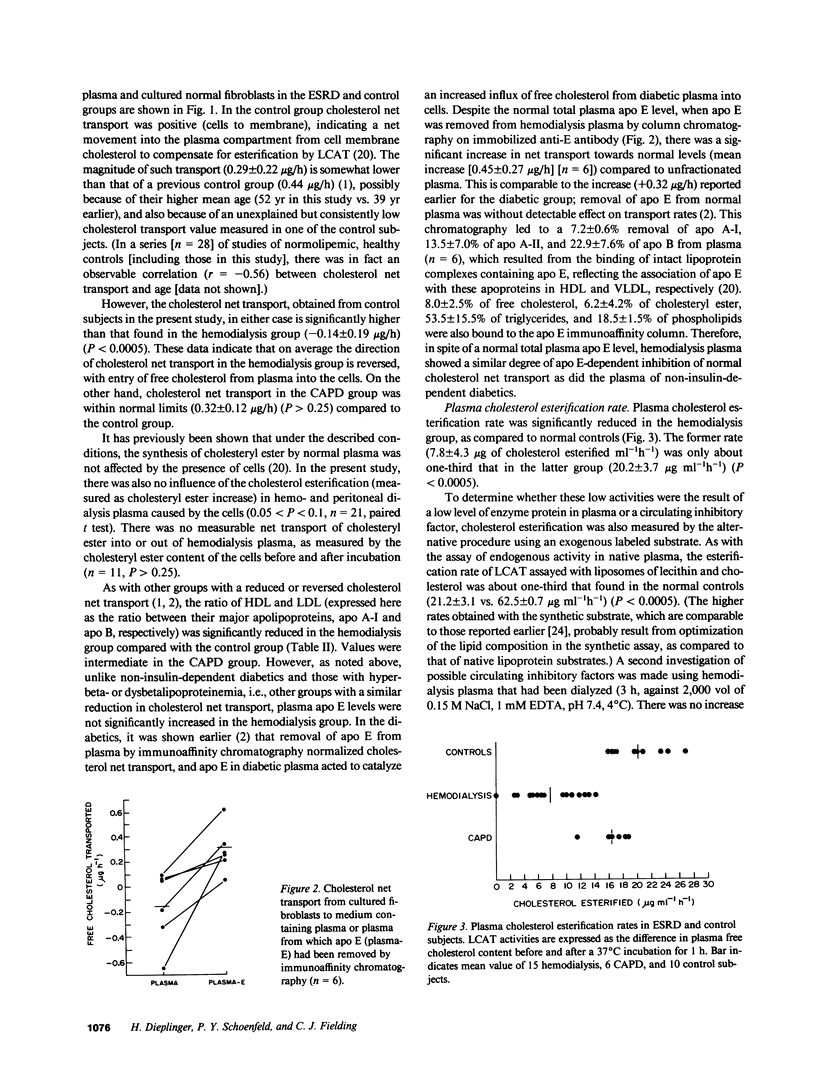
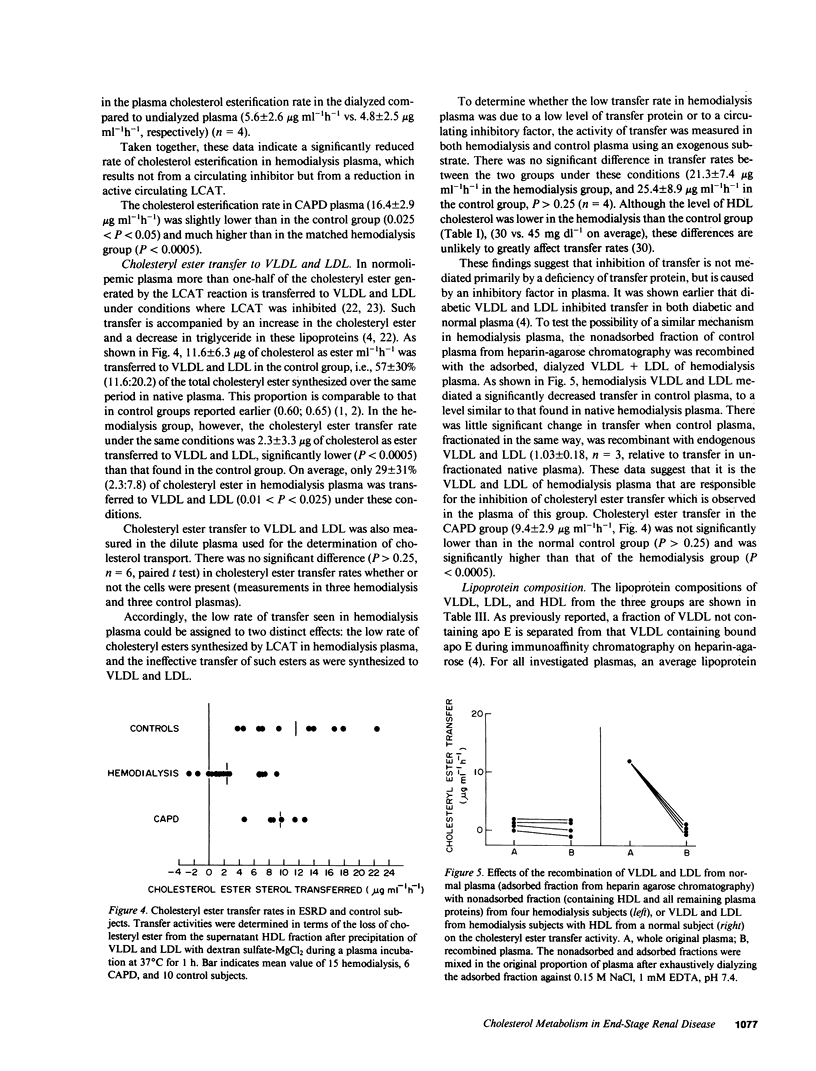
Plasma cholesterol metabolism was investigated in normotriglyceridemic patients with end-stage renal disease treated by hemo- or continuous ambulatory peritoneal dialysis (CAPD), and compared with that in a control group with normal renal function. A reversed net transport of free cholesterol from plasma to cultured fibroblasts, as well as greatly reduced levels of plasma cholesterol esterification and cholesterol ester transfer rates to low and very low density lipoproteins (LDL and VLDL), was found in the hemodialysis group compared to the controls. The LDL and VLDL contained increased amounts of free cholesterol and inhibited cholesterol ester transfer when recombined with control plasma. The LDL triglyceride content was doubled in the hemodialysis group, whereas cholesterol esters were decreased. Patients treated by CAPD, in marked contrast, had cholesterol metabolic rates that were within the normal range, as well as normal lipoprotein composition.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aron L., Jones S., Fielding C. J. Human plasma lecithin-cholesterol acyltransferase. Characterization of cofactor-dependent phospholipase activity. J Biol Chem. 1978 Oct 25;253(20):7220–7226. [PubMed] [Google Scholar]
- Bagdade J. D., Porte D., Jr, Bierman E. L. Hypertriglyceridemia. A metabolic consequence of chronic renal failure. N Engl J Med. 1968 Jul 25;279(4):181–185. doi: 10.1056/NEJM196807252790403. [DOI] [PubMed] [Google Scholar]
- Brunzell J. D., Albers J. J., Haas L. B., Goldberg A. P., Agadoa L., Sherrard D. J. Prevalence of serum lipid abnormalities in chronic hemodialysis. Metabolism. 1977 Aug;26(8):903–910. doi: 10.1016/0026-0495(77)90009-9. [DOI] [PubMed] [Google Scholar]
- Cheung M. C., Albers J. J. The measurement of apolipoprotein A-I and A-II levels in men and women by immunoassay. J Clin Invest. 1977 Jul;60(1):43–50. doi: 10.1172/JCI108767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fielding C. J., Fielding P. E. Evidence for a lipoprotein carrier in human plasma catalyzing sterol efflux from cultured fibroblasts and its relationship to lecithin:cholesterol acyltransferase. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3911–3914. doi: 10.1073/pnas.78.6.3911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fielding C. J., Fielding P. E. Regulation of human plasma lecithin:cholesterol acyltransferase activity by lipoprotein acceptor cholesteryl ester content. J Biol Chem. 1981 Mar 10;256(5):2102–2104. [PubMed] [Google Scholar]
- Fielding C. J., Frohlich J., Moser K., Fielding P. E. Promotion of sterol efflux and net transport by apolipoprotein E in lecithin:cholesterol acyltransferase deficiency. Metabolism. 1982 Oct;31(10):1023–1028. doi: 10.1016/0026-0495(82)90146-9. [DOI] [PubMed] [Google Scholar]
- Fielding C. J., Reaven G. M., Fielding P. E. Human noninsulin-dependent diabetes: identification of a defect in plasma cholesterol transport normalized in vivo by insulin and in vitro by selective immunoadsorption of apolipoprotein E. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6365–6369. doi: 10.1073/pnas.79.20.6365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fielding C. J., Reaven G. M., Liu G., Fielding P. E. Increased free cholesterol in plasma low and very low density lipoproteins in non-insulin-dependent diabetes mellitus: its role in the inhibition of cholesteryl ester transfer. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2512–2516. doi: 10.1073/pnas.81.8.2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fielding C. J. The origin and properties of free cholesterol potential gradients in plasma, and their relation to atherogenesis. J Lipid Res. 1984 Dec 15;25(13):1624–1628. [PubMed] [Google Scholar]
- Fielding P. E., Fielding C. J. A cholesteryl ester transfer complex in human plasma. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3327–3330. doi: 10.1073/pnas.77.6.3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fielding P. E., Fielding C. J., Havel R. J., Kane J. P., Tun P. Cholesterol net transport, esterification, and transfer in human hyperlipidemic plasma. J Clin Invest. 1983 Mar;71(3):449–460. doi: 10.1172/JCI110789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flamenbaum W. Metabolic consequences of antihypertensive therapy. Ann Intern Med. 1983 May;98(5 Pt 2):875–880. doi: 10.7326/0003-4819-98-5-875. [DOI] [PubMed] [Google Scholar]
- Glasson P., Leski M., Favre H. Dialyse péritoneéale chronique ambulatoire. Schweiz Med Wochenschr. 1981 Aug 22;111(34):1232–1238. [PubMed] [Google Scholar]
- Goldberg A. P., Harter H. R., Patsch W., Schechtman K. B., Province M., Weerts C., Kuisk I., McCrate M. M., Schonfeld G. Racial differences in plasma high-density lipoproteins in patients receiving hemodialysis. A possible mechanism for accelerated atherosclerosis in white men. N Engl J Med. 1983 May 26;308(21):1245–1252. doi: 10.1056/NEJM198305263082101. [DOI] [PubMed] [Google Scholar]
- Green D., Stone N. J., Krumlovsky F. A. Putative atherogenic factors in patients with chronic renal failure. Prog Cardiovasc Dis. 1983 Sep-Oct;26(2):133–144. doi: 10.1016/0033-0620(83)90027-0. [DOI] [PubMed] [Google Scholar]
- Heider J. G., Boyett R. L. The picomole determination of free and total cholesterol in cells in culture. J Lipid Res. 1978 May;19(4):514–518. [PubMed] [Google Scholar]
- Ihm J., Quinn D. M., Busch S. J., Chataing B., Harmony J. A. Kinetics of plasma protein-catalyzed exchange of phosphatidylcholine and cholesteryl ester between plasma lipoproteins. J Lipid Res. 1982 Dec;23(9):1328–1341. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lindner A., Charra B., Sherrard D. J., Scribner B. H. Accelerated atherosclerosis in prolonged maintenance hemodialysis. N Engl J Med. 1974 Mar 28;290(13):697–701. doi: 10.1056/NEJM197403282901301. [DOI] [PubMed] [Google Scholar]
- Lowrie E. G., Lazarus J. M., Hampers C. L., Merrill J. P. Editoral: Cardiovascular disease in dialysis patients. N Engl J Med. 1974 Mar 28;290(13):737–738. doi: 10.1056/NEJM197403282901311. [DOI] [PubMed] [Google Scholar]
- Miller K. W., Small D. M. Surface-to-core and interparticle equilibrium distributions of triglyceride-rich lipoprotein lipids. J Biol Chem. 1983 Nov 25;258(22):13772–13784. [PubMed] [Google Scholar]
- Nestel P. J., Fidge N. H., Tan M. H. Increased lipoprotein-remnant formation in chronic renal failure. N Engl J Med. 1982 Aug 5;307(6):329–333. doi: 10.1056/NEJM198208053070601. [DOI] [PubMed] [Google Scholar]
- Pagnan A., Havel R. J., Kane J. P., Kotite L. Characterization of human very low density lipoproteins containing two electrophoretic populations: double pre-beta lipoproteinemia and primary dysbetalipoproteinemia. J Lipid Res. 1977 Sep;18(5):613–622. [PubMed] [Google Scholar]
- Ramos J. M., Gokal R., Siamopolous K., Ward M. K., Wilkinson R., Kerr D. N. Continuous ambulatory peritoneal dialysis: three years' experience. Q J Med. 1983 Spring;52(206):165–186. [PubMed] [Google Scholar]
- Ramos J. M., Heaton A., McGurk J. G., Ward M. K., Kerr D. N. Sequential changes in serum lipids and their subfractions in patients receiving continuous ambulatory peritoneal dialysis. Nephron. 1983;35(1):20–23. doi: 10.1159/000183039. [DOI] [PubMed] [Google Scholar]
- Rapoport J., Aviram M., Chaimovitz C., Brook J. G. Defective high-density lipoprotein composition in patients on chronic hemodialysis. A possible mechanism for accelerated atherosclerosis. N Engl J Med. 1978 Dec 14;299(24):1326–1329. doi: 10.1056/NEJM197812142992402. [DOI] [PubMed] [Google Scholar]
- Rostand S. G., Gretes J. C., Kirk K. A., Rutsky E. A., Andreoli T. E. Ischemic heart disease in patients with uremia undergoing maintenance hemodialysis. Kidney Int. 1979 Nov;16(5):600–611. doi: 10.1038/ki.1979.170. [DOI] [PubMed] [Google Scholar]
- Schernthaner G., Kostner G. M., Dieplinger H., Prager R., Mühlhauser I. Apolipoproteins (A-I, A-II, B), Lp(a) lipoprotein and lecithin: cholesterol acyltransferase activity in diabetes mellitus. Atherosclerosis. 1983 Dec;49(3):277–293. doi: 10.1016/0021-9150(83)90139-9. [DOI] [PubMed] [Google Scholar]
- Shelburne F. A., Quarfordt S. H. The interaction of heparin with an apoprotein of human very low density lipoprotein. J Clin Invest. 1977 Oct;60(4):944–950. doi: 10.1172/JCI108849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trezzi E., Calvi C., Roma P., Catapano A. L. Subfractionation of human very low density lipoproteins by heparin-Sepharose affinity chromatography. J Lipid Res. 1983 Jun;24(6):790–795. [PubMed] [Google Scholar]


