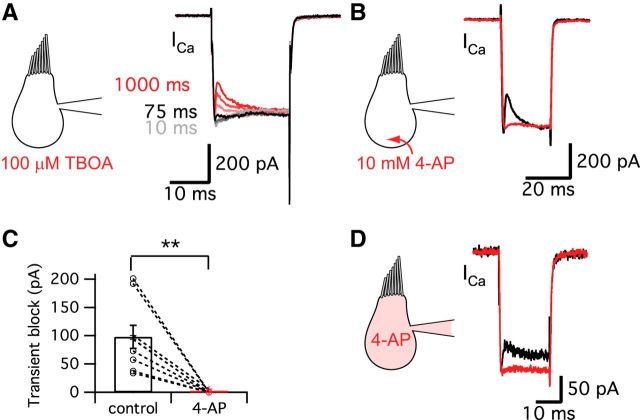
Figure 5.
Glutamate transporter and K+ currents do not contribute to the transient block. A, Ca2+ currents were evoked in hair cells held at −90 mV using a pre-depolarization to −60 mV for 10–1000 ms and then by a 20-ms-long pulse to −30 mV (same protocol as Fig. 2A). The Ca2+ currents for the 20 ms pulses are shown superimposed. The transient block of the Ca2+ currents was not blocked by 100 μm TBOA, a specific blocker of glutamate transporters (n = 6). The different colors represent different durations of the pre-depolarization. The Ca2+ current started showing the transient block within ∼75 ms of the pre-depolarization, which was very similar to control (Fig. 2C). B, 4-AP removed the transient block of the Ca2+ current. However, this effect may not be related with the blocking of K+ currents, but rather is probably due to the H+ capturing ability of 4-AP and its possible disruption of normal vesicle pH. C, 10 mm 4-AP significantly decreased the amplitude of transient block from 98 ± 20 pA to 0.6 ± 0.6 pA (n = 9). **p < 0.01, paired t test. Open circles indicate individual pairs. D, When hair cells were depolarized from −60 to −30 mV for 20 ms, 10 mm 4-AP in the patch pipette solution significantly decreased the transient block from 52.0 ± 9.4 pA (black) to 2.2 ± 2.1 pA (red, n = 9, p = 0.0011, paired t test). Control recordings were performed within 27 ± 7 s (n = 9) after break-in and the recordings of 4-AP effects were done at 4 min 39 ± 30 s (n = 9) after break-in to whole-cell mode.