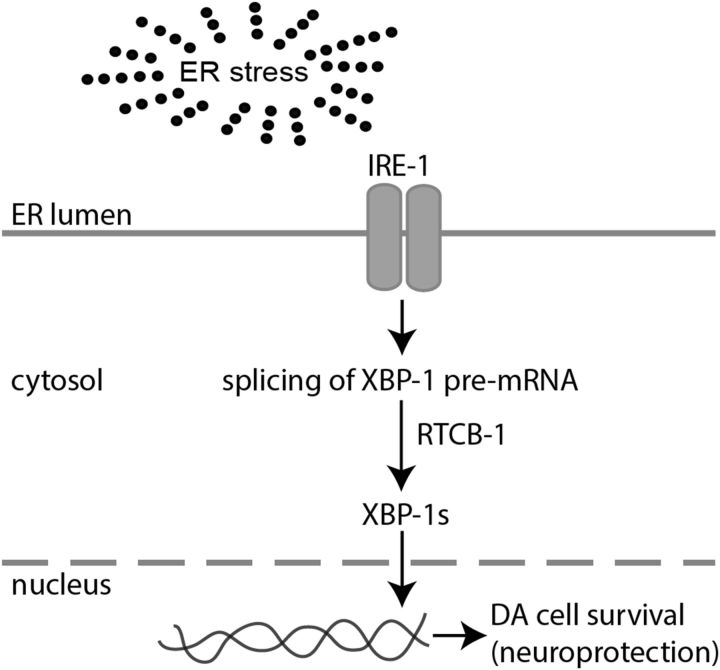
Figure 6.
Proposed model for RTCB-1-mediated DA neuroprotection in C. elegans. This tentative model describes our current understanding of the underlying mechanism involved in RTCB-1-induced neuroprotection against DA neuron cell death caused by various stressors. We have determined previously that RTCB-1 protects cells from α-syn protein misfolding or α-syn-induced neuronal toxicity. It is possible that, during ER stress caused by accumulation of such toxic misfolded or unwanted proteins, RTCB-1 acts as an RNA modifier that regulates the splicing of the XBP-1 transcription factor in UPR. This in turn activates downstream factors, such as ER chaperones and the hexosamine biosynthetic pathway, and stimulates cytoprotective mechanisms to reestablish protein homeostasis (Denzel et al., 2014). In our C. elegans PD model, cells depleted with rtcb-1 might block the UPR pathway because of a failure in xbp-1 splicing, leading to cell death or DA neuronal death caused by ER overload. Alternatively, overexpression of RTCB-1 might lead to prolonged xbp-1 splicing, thereby enhancing proteostasis activity, maintaining ER stress response, and thus protecting against neuronal damage caused by α-syn. Based on our neurodegeneration and splicing assay, the association between RTCB-1 and XBP-1 could be direct or indirect in the regulation of the UPR pathway.