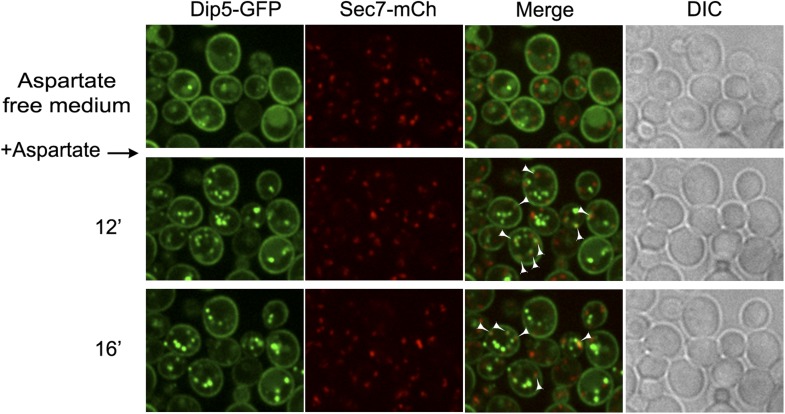
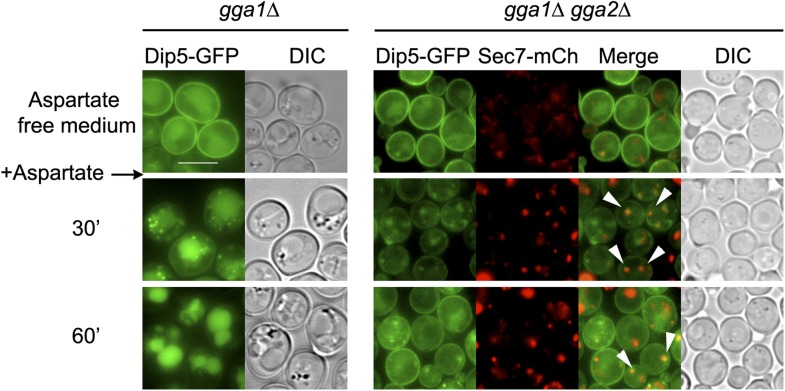
Figure 4. Jen1 transits through the TGN during its endocytosis and requires Rod1 for exit from the TGN to the vacuole.
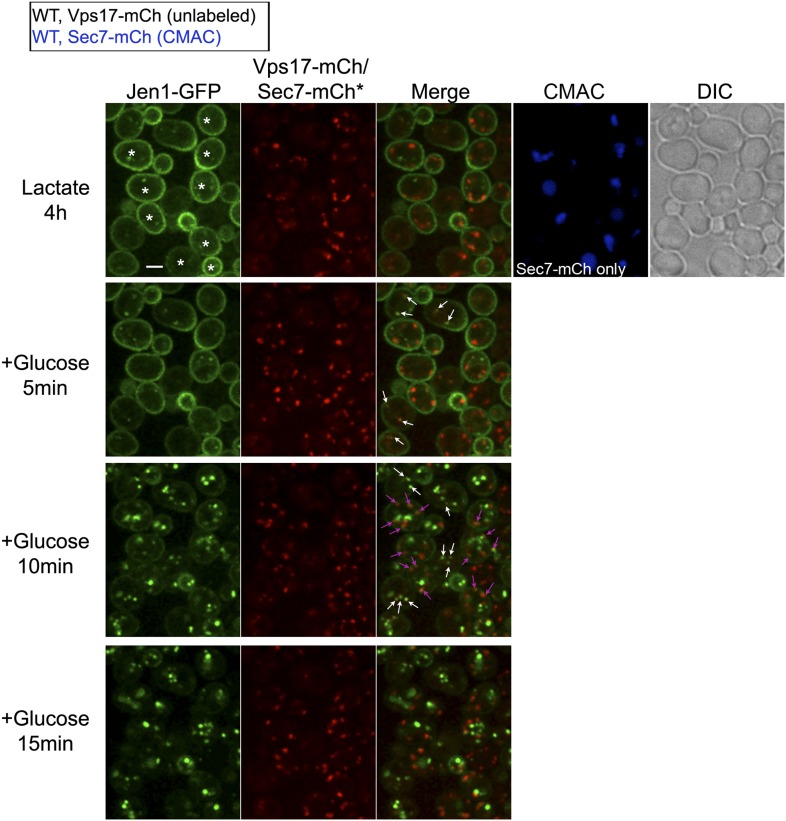
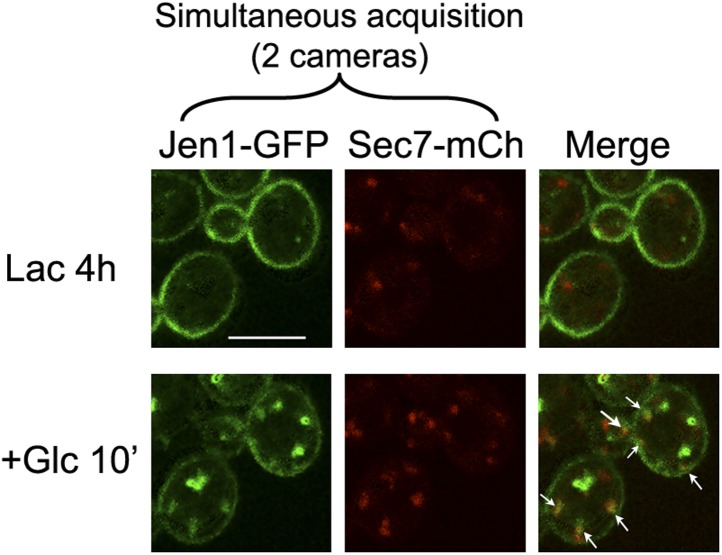
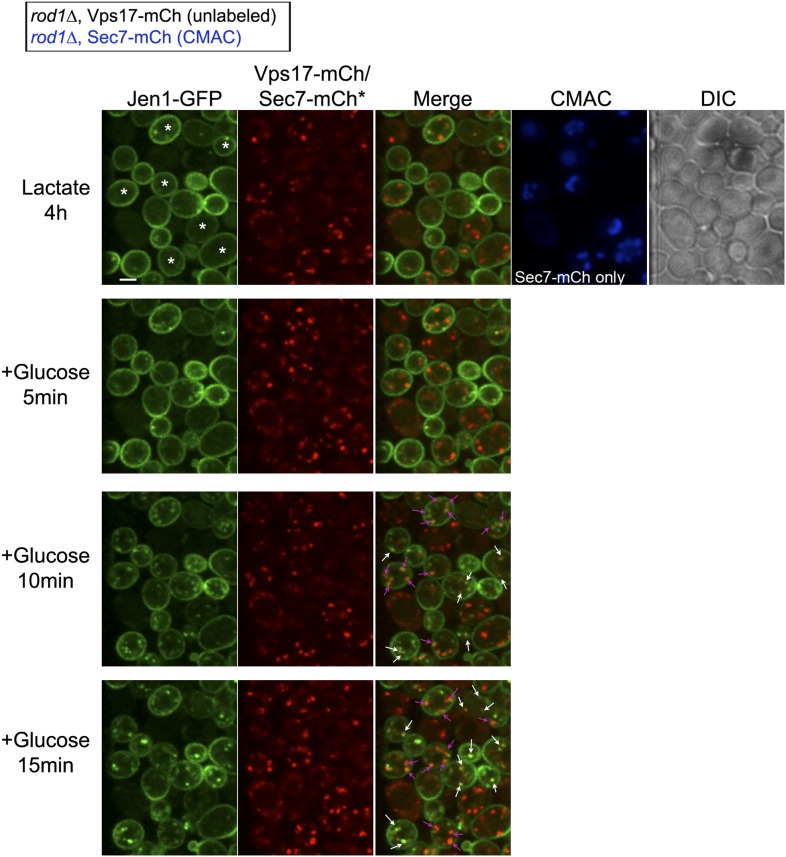
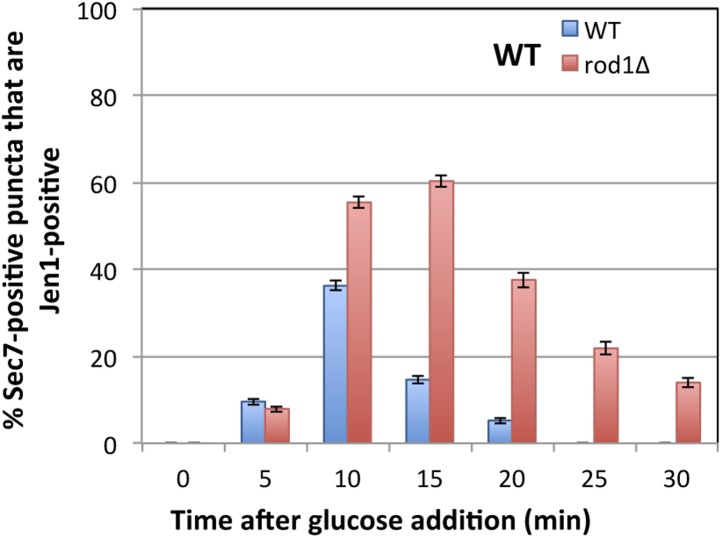
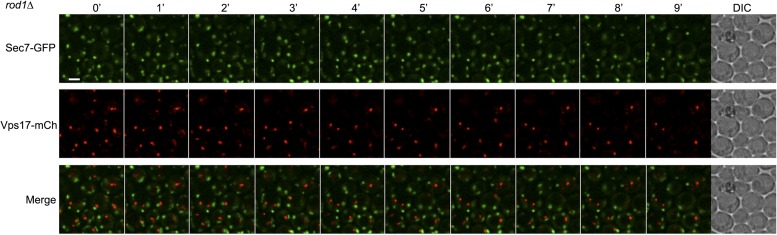
(A) Jen1 co-localizes with the TGN marker, Sec7-mCh, during its trafficking to the vacuole in wild-type cells. WT cells expressing Jen1-GFP and either Vps17-mCh (ySL1168), a marker of the early endosomal compartment, or Sec7-mCh (ySL1165) were grown on lactate medium. Of note, little or no co-localization was observed between Sec7-mCh and Vps17-GFP (Figure 4—figure supplement 1). The Sec7-mCh-expressing cells were labeled with CMAC and were co-injected with the Vps17-mCh-expressing cells into the microfluidics device in lactate medium, before glucose was added. Cells have been cropped to show only one Vps17-mCh expressing cell (left) or one Sec7-mCh expressing cell (right). The uncropped picture is displayed in Figure 4—figure supplement 2, and the full video in Video 6. Co-localization events between Jen1-GFP and either Vps17-mCh or Sec7-mCh are indicated with white arrows or pink arrows, respectively. Scale bar = 2.5 µm. Of note, the co-localization of another transporter, Dip5, with Sec7-mCh after its endocytosis was also observed (see Figure 4—figure supplement 3). (B) Jen1 also co-localizes with the TGN marker, Sec7-mCh, after internalization in the rod1Δ mutant. rod1Δ cells expressing Jen1-GFP and either Vps17-mCh (ySL1177), an endosomal marker, or Sec7-mCh (ySL1176) were grown on lactate medium. As in panel A, the Sec7-mCh-expressing cells were labeled with CMAC and were co-injected with Vps17-mCh-expressing cells into the microfluidics device in lactate medium, before glucose was added. Cells have been cropped to show only one Vps17-mCh expressing cell (left) or one Sec7-mCh expressing cell (right). The uncropped picture is displayed in Figure 4—figure supplement 4, and the full video in Video 7. Co-localization events between Jen1-GFP and either Vps17-mCh or Sec7-mCh are indicated with white arrows or pink arrows, respectively. Scale bar = 2.5 µm. (C) Quantification of the co-localization of Jen1-GFP puncta with either Vps17-mCh or Sec7-mCh puncta in WT (top) or rod1Δ (bottom) cells (20 cells, n = 3). Jen1 co-localizes successively with Vps17 and Sec7. Furthermore, Jen1 co-localizes more robustly with Sec7-mCh in the rod1Δ mutant. See also Figure 4—figure supplement 5. (D) Schematic showing the place of action of the VFT/GARP complex, Ypt6 and the GGA proteins in endosome-to-Golgi trafficking. (E) Deletions of VPS52 (VFT/GARP complex), YPT6 or genes encoding the Ypt6 GEF complex (RGP1 and RIC1) abolish Jen1 trafficking to the vacuole. Lactate-grown WT (ySL1150), vps52Δ (ySL1369), ypt6Δ (ySL1175), ric1Δ (ySL1630) and rgp1Δ (ySL1631) cells expressing Jen1-GFP were imaged before or after the addition of glucose at the indicated time. At t = 30 min Glc treatment, CMAC staining was used to visualize the localization of the vacuole. Scale bar = 5 µm. (F) Deletions of VPS52 or YPT6 prevent the vacuolar degradation of Jen1-GFP. Crude extracts from lactate-grown WT (ySL1150), vps52Δ (ySL1369) and ypt6Δ (ySL1175) cells expressing Jen1-GFP were prepared at the indicated times before and after glucose addition, and were immunoblotted with anti-GFP antibodies to reveal the full-length Jen1-GFP and its degradation product (free GFP). (G) Deletion of YPT6 abrogates Jen1 co-localization with Sec7-mCh. Lactate-grown WT cells (ySL1165) or ypt6Δ cells (ySL1526) expressing Jen1-GFP and Sec7-mCh were injected in a microfluidics device and imaged before (Lactate) and 15 min after glucose addition. Co-localization between Jen1-GFP and Sec7-mCh is indicated with arrowheads. (H) Deletions of GGA1 and GGA2, encoding redundant Golgi-localized clathrin adaptor proteins, alter Jen1 trafficking to the vacuole. Strains gga1Δgga2Δ (ySL1307) or gga1Δ gga2Δ expressing Gga2-HA (ySL1308), used as a positive control, both expressing Jen1-GFP were grown on lactate medium, and imaged before and after the addition of glucose at the indicated times. The gga1Δ gga2Δ cells also express Sec7-mCherry, which allows evaluating of Jen1-GFP co-localization with the TGN (arrowheads). Scale bar = 5 µm. (I) Quantification of the co-localization of Jen1-GFP puncta with Sec7-mCh puncta in gga1Δgga2Δ (ySL1307) cells or gga1Δ gga2Δ cells expressing Gga2-HA (ySL1619) (20 cells, n = 3) over time after glucose addition. Deletion of the GGA genes leads to a transient accumulation of Jen1 at the TGN.