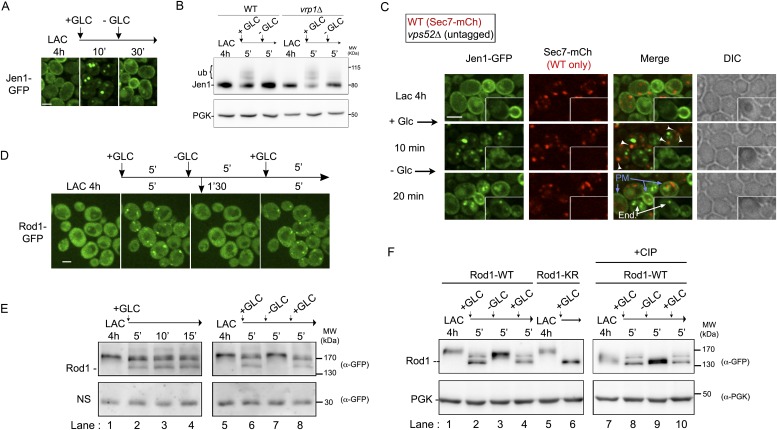
Figure 6. The control of Jen1 trafficking at the TGN allows the recycling of internalized transporters back to the cell membrane.
(A) Jen1 endocytosis is reversible upon glucose removal. Lactate-grown WT cells expressing Jen1-GFP (ySL1150) were injected into a microfluidics device in lactate medium. Cells were then imaged over time, before and after 10 min glucose addition, and then 20 min after glucose removal. Scale bar = 2.5 µm. (B) The persistence of the glucose signal is required to maintain Jen1 ubiquitylation. Lactate-grown WT cells expressing Jen1-GFP (ySL1150) were treated with glucose for 5 min. Cells were then briefly centrifuged and incubated back into lactate medium for 5 min. Crude extracts were prepared at each step and were immunoblotted with the indicated antibodies. The high-molecular weight species of Jen1 appearing upon glucose addition correspond to ubiquitylated Jen1 (see Becuwe et al., 2012b) (see also Figure 2B). The same extracts prepared from vrp1Δ cells show that Jen1 deubiquitylation observed in WT cells is not due to Jen1 degradation but to an active deubiquitylation process. (C) Lactate-grown WT (ySL1165) cells expressing both Jen1-GFP and Sec7-mCh, and vps52Δ (ySL1369) cells expressing only Jen1-GFP were co-injected into the microfluidics device in lactate medium, before glucose was added for 10 min and then removed. Cells were imaged at the indicated times. Arrowheads indicate examples of co-localization of Jen1-GFP and Sec7-mCh in WT cells, and arrows show the plasma membrane localization of Jen1-GFP after glucose removal in WT cells. Note the polarized distribution of Jen1-GFP after recycling in WT cells, but not in vps52Δ cells (inset). PM, plasma membrane; end: endosomes. Scale bar = 2.5 µm. See also Video 8. (D) Jen1 recycling correlates with the loss of Rod1 localization to the TGN. Lactate-grown cells (ySL542) expressing Rod1-GFP were injected into a microfluidics device in lactate medium. Cells were then subjected to 5-min pulses of glucose addition/removal and imaged simultaneously. Only the first three cycles are shown here. Scale bar = 2.5 µm. See also Video 9. (E–F) Dynamics of Rod1 post-translational modifications upon glucose/lactate cycles. Lactate-grown WT cells expressing a plasmid-borne Rod1-GFP were treated with glucose for 15′, or subjected to glucose addition/removal as indicated. Crude extracts were prepared at each step and were immunoblotted with the indicated antibodies. A non-specific (NS) cross-reacting band is used as a loading control. The high-molecular weight species of Rod1-GFP observed in the lactate samples correspond to phosphorylation (panel E, lanes 1, 5 and 7), because phosphatase treatment (CIP) abolishes the migration shift (panel F, lanes 7 and 9). The glucose-induced doublet (panel E, lanes 2, 3, 4, 6 and 8) corresponds to ubiquitylated (higher band) and non-ubiquitylated (lower band) forms of Rod1, because mutation of its ubiquitylation sites (using a plasmid encoding Rod1-KR) abolishes the migration shift (panel F, lane 6), as described previously (Becuwe et al., 2012b). PGK is used as a loading control in panel F.