Abstract
Immune factors are implicated in normal brain development and in brain disorder pathogenesis. Pathogen infection and food antigen penetration across gastrointestinal barriers are means by which environmental factors might affect immune-related neurodevelopment. Here, we test if gastrointestinal inflammation is associated with schizophrenia and therefore, might contribute to bloodstream entry of potentially neurotropic milk and gluten exorphins and/or immune activation by food antigens. IgG antibodies to Saccharomyces cerevisiae (ASCA, a marker of intestinal inflammation), bovine milk casein, wheat-derived gluten, and 6 infectious agents were assayed. Cohort 1 included 193 with non-recent onset schizophrenia, 67 with recent onset schizophrenia and 207 non-psychiatric controls. Cohort 2 included 103 with first episode schizophrenia, 40 of whom were antipsychotic-naïve. ASCA markers were significantly elevated and correlated with food antigen antibodies in recent onset and non-recent onset schizophrenia compared to controls (p ≤ 0.00001–0.004) and in unmedicated individuals with first episode schizophrenia compared to those receiving antipsychotics (p ≤ 0.05–0.01). Elevated ASCA levels were especially evident in non-recent onset females (p ≤ 0.009), recent onset males (p ≤ 0.01) and in antipsychotic-naïve males (p ≤ 0.03). Anti-food antigen antibodies were correlated to antibodies against Toxoplasma gondii, an intestinally-infectious pathogen, particularly in males with recent onset schizophrenia (p ≤ 0.002). In conclusion, gastrointestinal inflammation is a relevant pathology in schizophrenia, appears to occur in the absence of but may be modified by antipsychotics, and may link food antigen sensitivity and microbial infection as sources of immune activation in mental illness.
Keywords: immunology, environment, food hypersensitivity, microbiology, mental disorder, intestine
1. Introduction
Immune factors are increasingly the focus of research that explores gene-environmental interactions underlying the pathophysiology of schizophrenia (Abazyan et al., 2010; Brown, 2011; Shi et al., 2009; Stefansson et al., 2009; Yolken and Torrey, 2008). Prenatal exposure to microbial pathogens triggers inflammatory processes that may affect fetal neurodevelopment (Brown, 2011; Yolken and Torrey, 2008). Food antigens also activate the immune response and provide another means by which environmental factors might impact immune-related neurodevelopment. Adult individuals with psychiatric disorders such as schizophrenia and bipolar disorder exhibit increased humoral immune responses to food and microbial antigens (Cascella et al., 2011; Dickerson et al., 2011; Dickerson et al., 2010; Dohan et al., 1972; Reichelt and Landmark, 1995; Severance et al., 2010a; Severance et al., 2010b; Yolken and Torrey, 2008).
Food-specific antibody responses result from the entry of food antigens into the general circulation, presumably because of gastrointestinal (GI) inflammation or otherwise compromised GI epithelial and/or endothelial barriers. Bovine milk caseins and wheat glutens are of particular interest in neuropsychiatric disorders, because peptides derived from both can act as ligands of opioid receptors peripherally and in the central nervous system (Cade et al., 1990; Dohan, 1979; Drysdale et al., 1982; Reichelt et al., 1981; Reichelt and Stensrud, 1998). GI epithelial barriers can also be penetrated during an infection of the gut by enteric viruses or other microorganisms that are acquired through oral ingestion.
To determine if individuals with schizophrenia might possess a GI barrier defect that enables the passage of potentially detrimental antigens into the systemic circulation, we measured antibodies to anti-Saccharomyces cerevisiae, a marker of intestinal inflammation that is used as a diagnostic aid in Crohn’s Disease (Ashorn et al., 2009; Desplat-Jego et al., 2007; Kotze et al.; Mallant-Hent et al., 2006; Oshitani et al., 2000). We then compared these measures to markers of exposure to food antigens and infectious agents in serum and plasma from individuals with non-recent onset and recent onset schizophrenia, medicated and unmedicated first episode schizophrenia, and controls who had no history of psychiatric illness. In these experiments, we found that GI inflammation may provide a common mechanism by which multiple sources of immune activation in schizophrenia might be linked.
2. Experimental/Materials and methods
2.1 Study participants
2.1.1 Cohort 1 - Sheppard Pratt Health System, Baltimore, MD, USA
One hundred and ninety-three individuals with non-recent onset schizophrenia, 67 individuals with a recent onset of schizophrenia and 207 individuals who had no history of psychiatric disorders were recruited for this study. The methods for identifying and characterizing individuals of diagnostic groups according to criteria defined by DSM-IV have been previously described (Dickerson et al., 2010; Severance et al., 2010a; Severance et al., 2011).
For individuals with non-recent onset schizophrenia, inclusion criteria were: DSM-IV diagnosis of schizophrenia, schizophreniform disorder or schizoaffective disorder; age between 18–65, inclusive; and currently receiving antipsychotic medications. For individuals with recent onset of schizophrenia, inclusion criteria were: DSM-IV diagnosis of schizophrenia, schizophreniform disorder or schizoaffective disorder; the onset of psychotic symptoms for the first time within the past 24 months, defined as the presence of a positive psychotic symptom of at least moderate severity that lasted through the day for several days or occurred several times a week; age between 18 and 45, inclusive; and voluntary admission to either the inpatient or day hospital program. Individuals without a history of psychiatric disorder were recruited from posted announcements and were screened to rule out current or past psychiatric disorders with the Structured Clinical Interview for DSM-IV Axis I Disorders (First, 1998). Control participants were between the ages of 20 and 60, inclusive. Individuals with a diagnosis of recent onset schizophrenia had an average duration of illness of 0.78±0.08 years, and those with non-recent onset schizophrenia had an average duration of illness of 20.98±0.83 years.
Exclusion criteria for all three groups included the following: any history of intravenous substance abuse; mental retardation; and clinically significant medical disorder that would affect cognitive performance. With respect to substance abuse, for the recent onset group, psychosis that occurred only in the context of substance abuse, intoxication or withdrawal was the exclusion criterion; for the non-recent onset schizophrenia and control groups, current substance abuse that occurred over the past one month was the exclusion criterion.
Basic demographic data of the three cohort 1study populations are shown in Table 1. Diagnostic groups differed significantly in age and sex in t-tests and chi-square tests. These variables were included in the multivariate analyses described below.
Table 1.
Demographic information
| n | Age Mean years +SEMa |
Female n (%) |
Male n (%) |
African American n (%) |
Caucasian/Other n (%) |
|
|---|---|---|---|---|---|---|
| Cohort 1 - Sheppard Pratt, Baltimore, MD, USA Controls (CO) |
207 | 32.07±0.80 | 151 (72.95) |
56 d (27.05) | 66f (31.88) |
141 (68.12) |
| Non-recent onset schizophrenia (SZ) | 193 | 42.01±0.85b | 79 (40.93) |
114 (59.07) |
102 (53.85) |
91 (47.15) |
| Recent onset schizophrenia (ROSZ) | 67 | 22.28±0.65c | 16 (23.88) |
51e (76.12) |
31g (46.27) |
36 (53.73) |
| Cohort 2 - University of Cologne, Cologne, Germany | ||||||
| First episode schizophrenia - medicated | 63 | 29.38±1.21 | 11 (17.5) |
52 (82.5) |
n/a | n/a |
| First episode schizophrenia - unmedicated | 40 | 29.73±1.47 | 13 (32.5) |
27 (67.5) |
n/a | n/a |
SEM refers to standard error of the mean
CO vs SZ t=−8.57, p≤0.00001
CO vs ROSZ t=6.76, p≤0.00001
CO,SZ,RO chi2=48.6, p≤0.0001
CO ROSZ chi2=51.2, p≤0.0001
CO,SZ,RO chi2=19.5, p≤0.0001
CO ROSZ chi2=4.58, p≤0.032
Blood samples were obtained by venipuncture, and plasma and serum separated and assessed for antibodies in the assays described below.
The studies were approved by the Institutional Review Boards (IRB) of the Sheppard Pratt Health System and the Johns Hopkins Medical Institution following established guidelines. All participants provided written informed consent after study procedures were explained. The work described was carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans.
2.1.2 Cohort 2 - University of Cologne, Cologne, Germany
The methods for identifying and characterizing the individuals with a first episode of schizophrenia according to criteria defined by DSM-IV have also been previously described (Leweke et al., 2004). Forty of these patients were antipsychotic-naïve and 63 patients were currently receiving antipsychotic medication. Demographic data regarding age and sex are also listed in Table 1. The region from which patients were recruited was generally homogenous regarding socioeconomic characteristics. Informed consent was obtained from all study participants and procedures for sample collection and analysis were approved by the ethics committee at the University of Cologne in accordance with the Declaration of Helsinki.
2.2 Laboratory procedures
Anti-Saccharomyces cerevisiae IgG antibodies (ASCA) were measured according to the manufacturer’s protocol using a commercially available kit (Orgentec, Mainz, Germany). IgG antibodies to bovine milk casein and wheat gluten were measured by ELISAs using previously described methods (Severance et al., 2010a). Whole casein was purchased from Sigma-Aldrich (St. Louis, MO, U.S.A.). Whole gluten was extracted from the wheat cultivar Cheyenne as previously described (Samaroo et al., 2010). In brief, for both the casein and gluten immunoassays, plate wells were incubated with 100ng protein in 50μl carbonate buffer (0.05M carbonate-bicarbonate, pH 9.6; Sigma-Aldrich, St. Louis, MO, U.S.A.) overnight at 4°C, and plates were blocked for 1 h at 37°C with 1% (wt/vol) human serum albumin (Sigma-Aldrich, St. Louis, MO, U.S.A.) in PBS. Plates were then incubated with samples diluted 1:200 in PBST for 2h at 37°C. Plates were washed and incubated with peroxidase-conjugated goat-anti-human IgG secondary antibodies for 30min at 37°C (Southern Biotech, Birmingham, AL, U.S.A.). A 2,2’-azino-di-(3-ethylbenzthiazoline-6-sulfonate) and 0.02% hydrogen peroxide solution (KPL Protein Research Products, Gaithersburg, MD, U.S.A.) was added for color development, and absorbance was measured at 405 nm, with a reference wavelength of 490 nm, in an automated microtiter plate reader (Molecular Devices, Menlo Park, CA, U.S.A.). Casein antibody measurements for a portion of the individuals tested in this study has been previously reported (Severance et al., 2010a).
Commercially available ELISA kits for measuring EBV IgG, Influenza A IgG, Influenza B IgG, Measles IgG, Rubella IgG, and T. gondii IgG were purchased from IBL America (Minneapolis, MN, U.S.A.) and/or IBL International GmbH (Hamburg, Germany). HSV-1 kits were purchased from Focus Diagnostics (Cypress, CA, U.S.A.). IgG levels to these infectious disease agents had been previously measured for a series of studies at the Stanley Division at Johns Hopkins.
2.3 Statistical analyses
For cohort 1, plate-to-plate variation was corrected by control mean-normalizing each plate so that the control individuals on any particular plate equaled a value of “1”, as previously described (Severance et al., 2010a). For cohort 2, because there were no control individuals, plates were mean-normalized so that the values for the medicated individuals were equal to “1”. In both cohorts, quantitative antibody levels to ASCA were compared using t-tests and two-tailed p-values. In cohort 1, multiple linear regressions corrected for age, sex and race were implemented to test for inter-correlations of ASCA, food antigen and infectious disease antigen IgG antibody levels. For cohort 2, multiple linear regressions corrected for age and sex were used to test for correlations among ASCA, casein and gluten in antipsychotic-positive versus antipsychotic-naive individuals. Regression values that equaled or exceeded 0.15 and p values less than 0.05 were considered significant. For both cohorts, we broke down the diagnostic groups according to sex and race and applied the same analyses. We also examined correlations of ASCA with age. Statistical analyses were performed with STATA version 11 (STATA Corp LP, College Station, Texas, U.S.A.).
3. Results
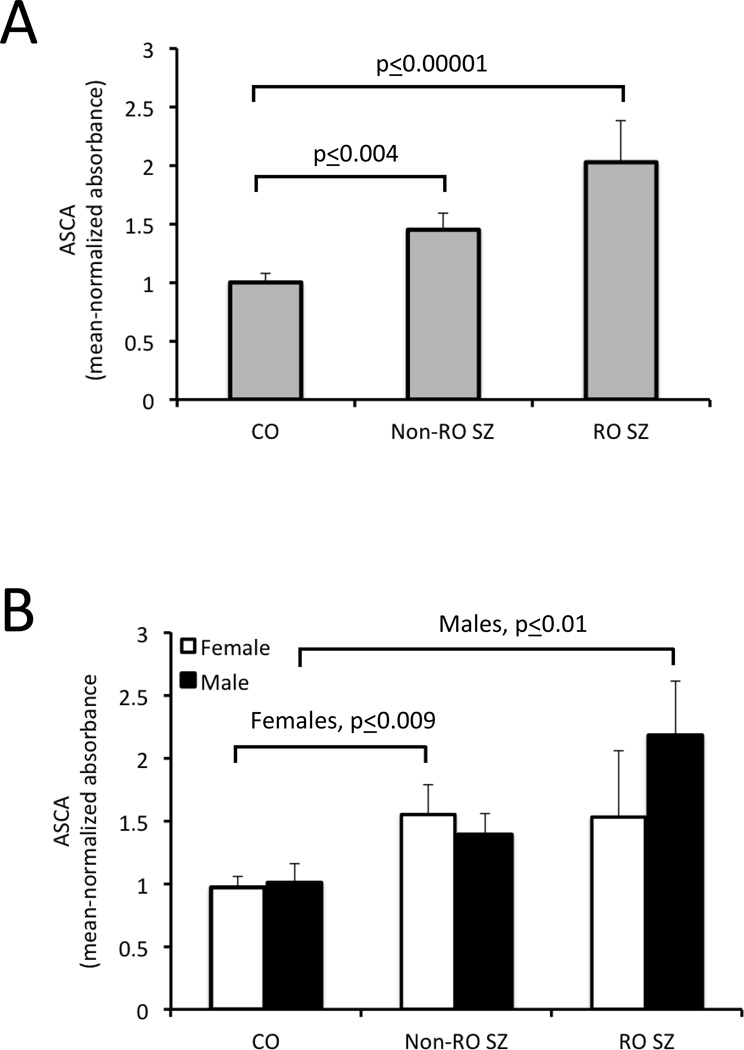
In 467 blood samples obtained from Sheppard Pratt Health System in Baltimore, MD, U.S.A., the levels of ASCA antibodies were significantly elevated in both non-recent onset (1.45±0.14, t=−2.86, p ≤ 0.004) and recent onset schizophrenia (2.03±0.35, t=−4.2, p ≤ 0.00001) compared to controls (1.00±0.08; Figure 1, Panel A). When the diagnostic groups were broken down according to sex, females in the non-recent onset group had significantly elevated antibody levels compared to control females, but levels between males in these two groups were not significantly different (Figure 1, Panel B). Conversely, in the recent onset group, males had statistically significant elevations in antibody levels compared to control males, whereas these differences were not detected between females in these two groups (Figure 1, Panel B).
Figure 1.
Quantitative ASCA IgG levels in individuals with schizophrenia compared to controls. CO refers to controls, Non-RO to non-recent onset, RO to recent onset and SZ to schizophrenia. P-values refer to the level of statistical significance following a two-tailed t-test. Panel A: Elevated ASCA IgG levels were found in individuals with Non-RO SZ and RO SZ compared to controls. Panel B: ASCA IgG levels were significantly elevated in females with Non-RO SZ compared to female controls and in males with RO SZ compared to male controls.
In the Sheppard Pratt cohort, levels of ASCA antibodies were significantly correlated with anti-casein and anti-gluten IgG in the non-recent onset group and with anti-gluten IgG in the recent onset group (Table 2). Following sex stratification, these correlations were significant in non-recent onset females compared to control females and in both sexes of the recent onset group compared to their respective control groups. Anti-casein and anti-gluten IgG levels were not significantly correlated with ASCA antibody levels in the non-psychiatric control group. Anti-casein and anti-gluten levels were significantly correlated to each other in all groups except for females with recent onset schizophrenia.
Table 2.
GI inflammation and food antigen antibody inter-correlations.
| Multiple linear regressions (corrected for age, sex, and race) |
||||
|---|---|---|---|---|
| ASCA:Casein | ASCA:Gluten | Casein:Gluten | ||
| n | R2, p-value | R2, p-value | R2, p-value | |
| Cohort 1 - Sheppard Pratt, Baltimore, MD, USA | ||||
| Control | 207 | 0.04, ns | 0.07, ns | 0.71, 0.00001 |
| Female | 151 | 0.025, ns | 0.08, ns | 0.70, 0.00001 |
| Male | 56 | 0.11, ns | 0.11, ns | 0.82, 0.00001 |
| Non-recent onset schizophrenia | 193 | 0.15, 0.00001 | 0.16, 0.00001 | 0.73, 0.00001 |
| Female | 79 | 0.18, 0.00001 | 0.23, .0002 | 0.80, 0.00001 |
| Male | 114 | 0.12, ns | 0.10, ns | 0.65, 0.00001 |
| Recent onset schizophrenia | 67 | 0.11, ns | 0.41, 0.00001 | 0.46, 0.00001 |
| Female | 16 | 0.60, ns | 0.84, 0.00001 | 0.41, ns |
| Male | 51 | 0.10, ns | 0.32, 0.001 | 0.45, 0.00001 |
| Cohort 2 - University of Cologne, Cologne, Germany | ||||
| First episode schizophrenia - unmedicated | 40 | 0.27, 0.01 | 0.21, 0.03 | 0.98, 0.00001 |
| Female | 13 | 0.45, 0.05 | 0.45, 0.05 | 0.99, 0.00001 |
| Male | 27 | 0.18, ns | 0.14, ns | 0.98, 0.00001 |
| First episode schizophrenia - medicated | 63 | 0.12, ns | 0.10, ns | 0.91, 0.00001 |
| Female | 11 | 0.07, ns | 0.09, ns | 0.98, 0.00001 |
| Male | 52 | 0.16, 0.01 | 0.11, ns | 0.89, 0.00001 |
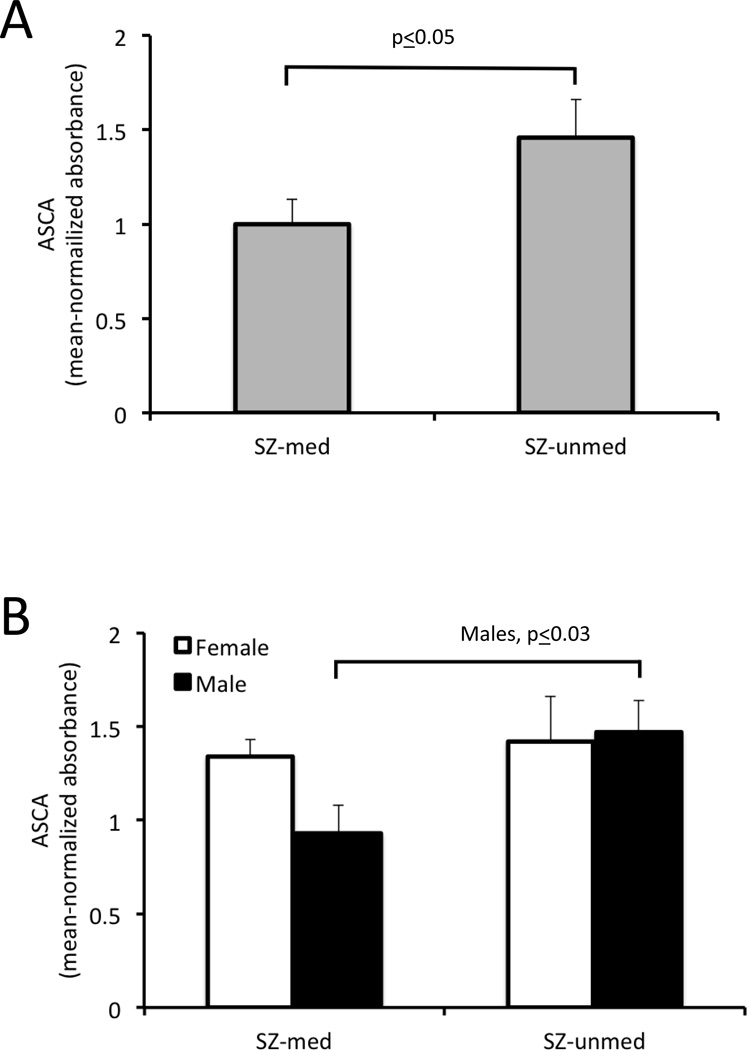
We then evaluated the effects of therapeutic treatment on these marker correlations using a cohort of 103 samples from Cologne, Germany, which was comprised of individuals with first episode schizophrenia, 40 of whom were antipsychotic-naive. In this group, individuals who were antipsychotic-naïve had ASCA levels that were significantly higher (1.46±0.20, t=−1.99, p ≤ 0.05) than those who received antipsychotic medications (1.0±0.13; Figure 2, Panel A). When these groups were broken down according to sex, only males who were antipsychotic naïve showed significantly elevated ASCA levels (Figure 2, Panel B). It was also only in the untreated group where anti-casein and anti-gluten IgG levels were significantly correlated with ASCA, and this finding was most apparent in women who were antipsychotic naïve (Table 2). ASCA and antibodies to these food antigens were not significantly correlated in those receiving antipsychotic medication except for a modest correlation of ASCA with anti-casein IgG in men of this group (Table 2). As with the Baltimore cohort, anti-casein and anti-gluten IgG levels were very highly correlated (Table 2).
Figure 2.
ASCA IgG levels in individuals with schizophrenia according to medication status. SZ-unmed refers to antipsychotic naïve schizophrenia, and SZ-med refers to antipsychotic-positive schizophrenia. P-values refer to the level of statistical significance following a two-tailed t-test. Panel A: ASCA IgG levels were elevated in individuals who are antipsychotic naïve compared to those who received these medications. Panel B: ASCA IgG levels were significantly elevated in males who were antipsychotic naïve compared to males who were antipsychotic-positive.
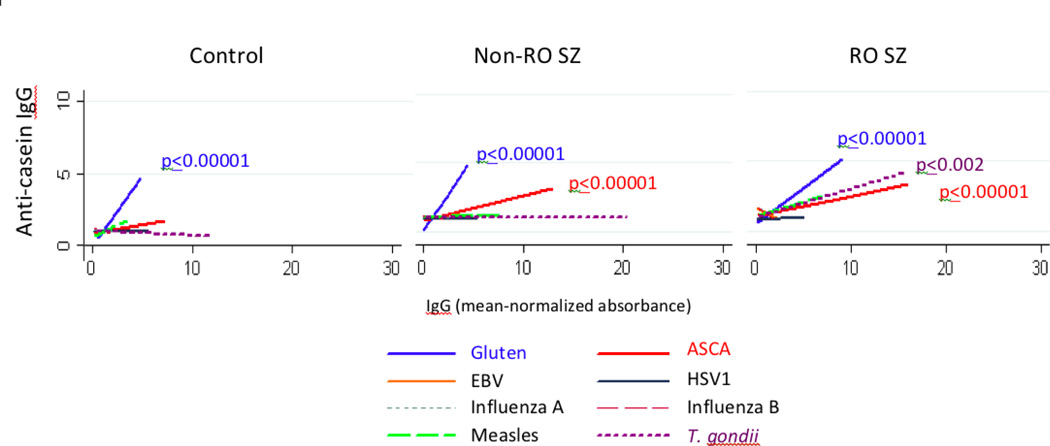
To determine if these correlations reflected a greater generalized immune activation, we reviewed previously collected cohort 1 data of antibodies directed at a variety of pathogenic microorganisms. With one exception, ASCA, anti-casein IgG and anti-gluten IgG levels were not strongly correlated with the infectious disease agent antibody levels in any diagnostic group (Figure 3). In the recent onset group, we observed a significant correlation of casein IgG with T. gondii IgG (R2=0.23, p ≤ 0.002), a finding that was specific to men in this group (R2=0.51, p ≤ 0.00001). Men with recent onset schizophrenia also showed significant correlations of gluten IgG with T. gondii IgG R2=00.24, p ≤ 0.0004).
Figure 3.
Correlations of GI inflammation, food antigen IgG and IgG to infectious disease agents in the diagnostic groups. Anti-casein IgG, anti-gluten IgG and ASCA antibody levels were compared to each other as well as to IgG of the infectious disease antigens. Anti-casein IgG used as the Y-axis component is shown as a representative chart and refers to antibody levels following mean-normalized absorbance. P-values refer to the level of statistical significance following a multiple linear regression that was corrected for age, sex and race.
In neither cohort did we find significant effects of race or age on ASCA antibody levels or ASCA correlations with other antigens.
4. Discussion
The goal of our investigation was to evaluate if intestinal inflammation might be connected to food antigen-associated immune activation in individuals with schizophrenia and secondarily, to determine if this link was affected by antipsychotic medication. GI inflammation was elevated and was correlated with food antigen antibodies only in the schizophrenia groups, suggesting that disease-associated inflammation is one means by which caseins and glutens may gain entry into the systemic circulation and/or generate a humoral immune response. The ASCA markers were elevated in antipsychotic-naïve individuals suggesting a prevalence of this type of inflammation early in the course of disease, even before treatments are implemented. We also found a possible association between T. gondii-generated intestinal inflammation and anti-food antigen antibodies in the recent onset patient group. Infection of the GI tract, therefore, may be one factor modulating the development of antibodies to milk caseins and wheat glutens.
Over the years, strong associations between GI pathologies and psychiatric disorders have been reported, yet it has been difficult to distinguish cause from effect and characterize how antipsychotics impact GI symptoms (Alander et al., 2005; Buscaino, 1953; Cascella et al., 2011; Eaton et al., 2004; Haug et al., 2002; Hemmings, 2004; Kalaydjian et al., 2006; Pynnonen et al., 2005; Reiter, 1926). Particularly pertinent to our findings are autopsy examinations reporting extensive inflammatory changes throughout the GI tract of patients with psychiatric disorders and studies that examine associations of celiac disease with schizophrenia (Buscaino, 1953; Cascella et al., 2011; Dohan, 1970; Eaton et al., 2004; Hemmings, 2004; Kalaydjian et al., 2006; Pynnonen et al., 2005; Reiter, 1926). Autopsy results from one study of 82 patients with schizophrenia indicated that 50% had gastritis, 88% enteritis and 92% colitis (Buscaino, 1953; Hemmings, 2004). Celiac disease, an established risk factor for the development of schizophrenia, is characterized by inflammatory damage to intestinal villi following immune reaction to ingested wheat gluten (Cascella et al., 2011; Eaton et al., 2004; Kalaydjian et al., 2006; Pynnonen et al., 2005). These studies collectively support that structural damage to the GI tract is present in schizophrenia, and our results document via a new measure of inflammation that GI pathologies are evident and may occur independently of antipsychotic medication.
First and second generation antipsychotics are typically associated with GI motility issues such as constipation and bowel obstruction, but are also likely to affect cytokine balance and other aspects of immune function (Dean, 2010; Dome et al., 2007; McNamara et al., 2011; Watanabe et al., 2010). In the Baltimore psychiatric groups, most of these individuals received antipsychotic medications at some point during their disease history and also at the time of the assessment, so it was not possible to evaluate whether medication status contributed to the GI-related correlations. Therefore, we included a study arm here specifically to address the medication issue through comparisons of individuals who were antipsychotic-naïve with those who received antipsychotics. We found no significant differences in anti-casein and anti-gluten IgG inter-associations between those who were medicated and those were medication-free; however, for the ASCA inflammation marker, it was predominately the untreated group that retained significantly elevated levels of the marker as well as a correlation with the anti-food antigen IgGs. This finding suggests that antipsychotic agents may affect the type or degree of GI inflammation identified with ASCA, but that the disease-associated inflammation is present before the start of pharmacological treatment.
We also evaluated antibody level correlations of the food antigens with those of the infectious disease antigens to determine if the documented immune activation could be the result of multiple types of antigens creating a non-specific, activated immune state. We found that the food-based antigens, while significantly and expectedly inter-correlated, were generally not significantly correlated with antibodies to the infectious agents, with one important exception: antibodies to T. gondii were significantly associated with anti-casein antibodies in the recent onset group of the Baltimore cohort. The protozoan, T. gondii, a neurotropic parasite, is of particular interest here, in light of its association with the development of schizophrenia and its use as a model of inflammatory bowel disease in experimental animals (Bereswill et al., 2010; Liesenfeld, 2002; Mortensen et al., 2007; Schreiner and Liesenfeld, 2009; Torrey et al., 2007; Xiao et al., 2009; Yolken et al., 2009). Findings from our study suggest that infection with this protozoan may create altered GI permeability, which in turn may result in increased absorption of partially digested casein or gluten peptides. T. gondii relevance to schizophrenia may therefore include its role as an agent of intestinal inflammation in addition to its role as a neurotropic pathogen.
Many of the patterns uncovered in this study were sex-specific; however, full interpretations of these associations are limited by the low number of women in the recent onset schizophrenia group (n=16) and in both groups of cohort 2 (n=11 medicated, n=13 antipsychotic naïve). Nevertheless, elevated ASCA antibody levels and correlations of GI inflammation with the casein and gluten antibodies were found specifically in women with non-recent onset schizophrenia, a group where we had sufficient numbers of both sexes (males, n=114; females, n=79). This finding reinforces that the pathophysiology of this disease and its treatment likely impact men and women differently. For example, one interpretation of these data are that GI inflammation in men is particularly prevalent early on and resolves over time, whereas women may have inflammation that persists throughout the course of the disease.
Overall, our results indicate that alterations in GI inflammation and permeability may contribute to the etiopathogenesis and/or symptomatology of schizophrenia. Genes may dictate those individuals who are especially susceptible to environmentally-induced barrier permeability issues. Some recently identified candidate genes that have particular relevance to mechanisms involving GI permeability and immune activation include vasoactive intestinal peptide receptor 2 (VIPR2), the major histocompatibility complex 2 (MHC2) and complement control-related genes (CSMD1 and CSMD2) (Havik et al., 2011; Shi et al., 2009; Stefansson et al., 2009; Vacic et al., 2011). Ultimately, an understanding of the interactions between intestinal inflammation and predisposing genetic factors may lead to new methods of identifying, treating and preventing psychotic disorders.
References
- Abazyan B, Nomura J, Kannan G, Ishizuka K, Tamashiro KL, Nucifora F, Pogorelov V, Ladenheim B, Yang C, Krasnova IN, Cadet JL, Pardo C, Mori S, Kamiya A, Vogel MW, Sawa A, Ross CA, Pletnikov MV. Prenatal interaction of mutant DISC1 and immune activation produces adult psychopathology. Biol Psychiatry. 2010;68(12):1172–1181. doi: 10.1016/j.biopsych.2010.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alander T, Svardsudd K, Johansson SE, Agreus L. Psychological illness is commonly associated with functional gastrointestinal disorders and is important to consider during patient consultation: a population-based study. BMC Med. 2005;3:8. doi: 10.1186/1741-7015-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashorn S, Valineva T, Kaukinen K, Ashorn M, Braun J, Raukola H, Rantala I, Collin P, Maki M, Luukkaala T, Iltanen S. Serological responses to microbial antigens in celiac disease patients during a gluten-free diet. J Clin Immunol. 2009;29(2):190–195. doi: 10.1007/s10875-008-9255-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bereswill S, Munoz M, Fischer A, Plickert R, Haag LM, Otto B, Kuhl AA, Loddenkemper C, Gobel UB, Heimesaat MM. Anti-inflammatory effects of resveratrol, curcumin and simvastatin in acute small intestinal inflammation. PLoS One. 2010;5(12):e15099. doi: 10.1371/journal.pone.0015099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AS. The environment and susceptibility to schizophrenia. Prog Neurobiol. 2011;93(1):23–58. doi: 10.1016/j.pneurobio.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buscaino V. Patologia extraneurale della schizofrenia. Fegato, tubo digerente, sistema reticolo-endoteliale. Acta neurologica. 1953;VIII:1–60. [Google Scholar]
- Cade R, Wagemaker H, Privette RM, Fregly MJ, Rogers J, Orlando J. The Effect of Dialysis and Diet on Schizophrenia. Psychiatry : A World Perspective. 1990;3(900):494–500. [Google Scholar]
- Cascella NG, Kryszak D, Bhatti B, Gregory P, Kelly DL, Mc Evoy JP, Fasano A, Eaton WW. Prevalence of celiac disease and gluten sensitivity in the United States clinical antipsychotic trials of intervention effectiveness study population. Schizophr Bull. 2011;37(1):94–100. doi: 10.1093/schbul/sbp055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean B. Understanding the role of inflammatory-related pathways in the pathophysiology and treatment of psychiatric disorders: evidence from human peripheral studies and CNS studies. Int J Neuropsychopharmacol. 2010;14(7):997–1012. doi: 10.1017/S1461145710001410. [DOI] [PubMed] [Google Scholar]
- Desplat-Jego S, Johanet C, Escande A, Goetz J, Fabien N, Olsson N, Ballot E, Sarles J, Baudon JJ, Grimaud JC, Veyrac M, Chamouard P, Humbel RL. Update on Anti-Saccharomyces cerevisiae antibodies, anti-nuclear associated anti-neutrophil antibodies and antibodies to exocrine pancreas detected by indirect immunofluorescence as biomarkers in chronic inflammatory bowel diseases: results of a multicenter study. World J Gastroenterol. 2007;13(16):2312–2318. doi: 10.3748/wjg.v13.i16.2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson F, Stallings C, Origoni A, Vaughan C, Khushalani S, Alaedini A, Yolken R. Markers of gluten sensitivity and celiac disease in bipolar disorder. Bipolar Disord. 2011;13(1):52–58. doi: 10.1111/j.1399-5618.2011.00894.x. [DOI] [PubMed] [Google Scholar]
- Dickerson F, Stallings C, Origoni A, Vaughan C, Khushalani S, Leister F, Yang S, Krivogorsky B, Alaedini A, Yolken R. Markers of gluten sensitivity and celiac disease in recent-onset psychosis and multi-episode schizophrenia. Biol Psychiatry. 2010;68(1):100–104. doi: 10.1016/j.biopsych.2010.03.021. [DOI] [PubMed] [Google Scholar]
- Dohan FC. Coeliac disease and schizophrenia. Lancet. 1970;1(7652):897–898. doi: 10.1016/s0140-6736(70)91729-0. [DOI] [PubMed] [Google Scholar]
- Dohan FC. Schizophrenia and neuroactive peptides from food. Lancet. 1979;1(8124):1031. doi: 10.1016/s0140-6736(79)92780-6. [DOI] [PubMed] [Google Scholar]
- Dohan FC, Martin L, Grasberger JC, Boehme D, Cottrell JC. Antibodies to wheat gliadin in blood of psychiatric patients: possible role of emotional factors. Biol Psychiatry. 1972;5(2):127–137. [PubMed] [Google Scholar]
- Dome P, Teleki Z, Kotanyi R. Paralytic ileus associated with combined atypical antipsychotic therapy. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(2):557–560. doi: 10.1016/j.pnpbp.2006.10.012. [DOI] [PubMed] [Google Scholar]
- Drysdale A, Deacon R, Lewis P, Olley J, Electricwala A, Sherwood R. A Peptide-Containing Fraction of Plasma from Schizophrenic-Patients Which Binds to Opiate Receptors and Induces Hyperreactivity in Rats. Neuroscience. 1982;7(6):1567–1573. doi: 10.1016/0306-4522(82)90265-2. [DOI] [PubMed] [Google Scholar]
- Eaton W, Mortensen PB, Agerbo E, Byrne M, Mors O, Ewald H. Coeliac disease and schizophrenia: population based case control study with linkage of Danish national registers. Bmj. 2004;328(7437):438–439. doi: 10.1136/bmj.328.7437.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders - Non-patient Edition (SCID I/NP) New York: Biometrics Research, New York State Psychiatric Institute; 1998. [Google Scholar]
- Haug TT, Mykletun A, Dahl AA. Are anxiety and depression related to gastrointestinal symptoms in the general population? Scand J Gastroenterol. 2002;37(3):294–298. doi: 10.1080/003655202317284192. [DOI] [PubMed] [Google Scholar]
- Havik B, Le Hellard S, Rietschel M, Lybaek H, Djurovic S, Mattheisen M, Muhleisen TW, Degenhardt F, Priebe L, Maier W, Breuer R, Schulze TG, Agartz I, Melle I, Hansen T, Bramham CR, Nothen MM, Stevens B, Werge T, Andreassen OA, Cichon S, Steen VM. The complement control-related genes CSMD1 and CSMD2 associate to schizophrenia. Biol Psychiatry. 2011;70(1):35–42. doi: 10.1016/j.biopsych.2011.01.030. [DOI] [PubMed] [Google Scholar]
- Hemmings G. Schizophrenia. Lancet. 2004;364(9442):1312–1313. doi: 10.1016/S0140-6736(04)17181-X. [DOI] [PubMed] [Google Scholar]
- Kalaydjian AE, Eaton W, Cascella N, Fasano A. The gluten connection: the association between schizophrenia and celiac disease. Acta Psychiatr Scand. 2006;113(2):82–90. doi: 10.1111/j.1600-0447.2005.00687.x. [DOI] [PubMed] [Google Scholar]
- Kotze LM, Nisihara RM, Utiyama SR, Kotze PG, Theiss PM, Olandoski M. Antibodies anti-Saccharomyces cerevisiae (ASCA) do not differentiate Crohn's disease from celiac disease. Arq Gastroenterol. 2010;47(3):242–245. doi: 10.1590/s0004-28032010000300006. [DOI] [PubMed] [Google Scholar]
- Leweke FM, Gerth CW, Koethe D, Klosterkotter J, Ruslanova I, Krivogorsky B, Torrey EF, Yolken RH. Antibodies to infectious agents in individuals with recent onset schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2004;254(1):4–8. doi: 10.1007/s00406-004-0481-6. [DOI] [PubMed] [Google Scholar]
- Liesenfeld O. Oral infection of C57BL/6 mice with Toxoplasma gondii: a new model of inflammatory bowel disease? . J Infect Dis. 2002;185(Suppl 1):S96–S101. doi: 10.1086/338006. [DOI] [PubMed] [Google Scholar]
- Mallant-Hent R, Mary B, von Blomberg E, Yuksel Z, Wahab PJ, Gundy C, Meyer GA, Mulder CJ. Disappearance of anti-Saccharomyces cerevisiae antibodies in coeliac disease during a gluten-free diet. Eur J Gastroenterol Hepatol. 2006;18(1):75–78. doi: 10.1097/00042737-200601000-00013. [DOI] [PubMed] [Google Scholar]
- McNamara RK, Jandacek R, Rider T, Tso P. Chronic risperidone normalizes elevated pro-inflammatory cytokine and C-reactive protein production in omega-3 fatty acid deficient rats. Eur J Pharmacol. 2011;652(1–3):152–156. doi: 10.1016/j.ejphar.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen PB, Norgaard-Pedersen B, Waltoft BL, Sorensen TL, Hougaard D, Yolken RH. Early infections of Toxoplasma gondii and the later development of schizophrenia. Schizophr Bull. 2007;33(3):741–744. doi: 10.1093/schbul/sbm009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshitani N, Hato F, Matsumoto T, Jinno Y, Sawa Y, Hara J, Nakamura S, Seki S, Arakawa T, Kitano A, Kitagawa S, Kuroki T. Decreased anti-Saccharomyces cerevisiae antibody titer by mesalazine in patients with Crohn's disease. J Gastroenterol Hepatol. 2000;15(12):1400–1403. doi: 10.1046/j.1440-1746.2000.02357.x. [DOI] [PubMed] [Google Scholar]
- Pynnonen PA, Isometsa ET, Verkasalo MA, Kahkonen SA, Sipila I, Savilahti E, Aalberg VA. Gluten-free diet may alleviate depressive and behavioural symptoms in adolescents with coeliac disease: a prospective follow-up case-series study. BMC Psychiatry. 2005;5:14. doi: 10.1186/1471-244X-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichelt KL, Hole K, Hamberger A, Saelid G, Edminson PD, Braestrup CB, Lingjaerde O, Ledaal P, Orbeck H. Biologically active peptide-containing fractions in schizophrenia and childhood autism. Adv Biochem Psychopharmacol. 1981;28:627–643. [PubMed] [Google Scholar]
- Reichelt KL, Landmark J. Specific Iga Antibody Increases in Schizophrenia. Biol Psychiatry. 1995;37(6):410–413. doi: 10.1016/0006-3223(94)00176-4. [DOI] [PubMed] [Google Scholar]
- Reichelt KL, Stensrud M. Increase in urinary peptides prior to the diagnosis of schizophrenia. Schizophr Res. 1998;34(3):211–213. doi: 10.1016/s0920-9964(98)00104-2. [DOI] [PubMed] [Google Scholar]
- Reiter P. Extrapyramidal motor disturbances in dementia praecox. Acta Psychiatrica et Neurologica (KjøBenhavn) 1926;1:287–304. [Google Scholar]
- Samaroo D, Dickerson F, Kasarda DD, Green PH, Briani C, Yolken RH, Alaedini A. Novel immune response to gluten in individuals with schizophrenia. Schizophr Res. 2010;118(1–l3):248–255. doi: 10.1016/j.schres.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiner M, Liesenfeld O. Small intestinal inflammation following oral infection with Toxoplasma gondii does not occur exclusively in C57BL/6 mice: review of 70 reports from the literature. Mem Inst Oswaldo Cruz. 2009;104(2):221–233. doi: 10.1590/s0074-02762009000200015. [DOI] [PubMed] [Google Scholar]
- Severance EG, Dickerson FB, Halling M, Krivogorsky B, Haile L, Yang S, Stallings CR, Origoni AE, Bossis I, Xiao J, Dupont D, Haasnoot W, Yolken RH. Subunit and whole molecule specificity of the anti-bovine casein immune response in recent onset psychosis and schizophrenia. Schizophr Res. 2010a;118(1–3):240–247. doi: 10.1016/j.schres.2009.12.030. [DOI] [PubMed] [Google Scholar]
- Severance EG, Dickerson FB, Viscidi RP, Bossis I, Stallings CR, Origoni AE, Sullens A, Yolken RH. Coronavirus immunoreactivity in individuals with a recent onset of psychotic symptoms. Schizophr Bull. 2011;37(1):101–107. doi: 10.1093/schbul/sbp052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severance EG, Dupont D, Dickerson FB, Stallings CR, Origoni AE, Krivogorsky B, Yang S, Haasnoot W, Yolken RH. Immune activation by casein dietary antigens in bipolar disorder. Bipolar Disord. 2010b;12(8):834–842. doi: 10.1111/j.1399-5618.2010.00879.x. [DOI] [PubMed] [Google Scholar]
- Shi J, Levinson DF, Duan J, Sanders AR, Zheng Y, Pe'er I, Dudbridge F, Holmans PA, Whittemore AS, Mowry BJ, Olincy A, Amin F, Cloninger CR, Silverman JM, Buccola NG, Byerley WF, Black DW, Crowe RR, Oksenberg JR, Mirel DB, Kendler KS, Freedman R, Gejman PV. Common variants on chromosome 6p22.1 are associated with schizophrenia. Nature. 2009;460(7256):753–757. doi: 10.1038/nature08192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefansson H, Ophoff RA, Steinberg S, Andreassen OA, Cichon S, Rujescu D, Werge T, Pietilainen OP, Mors O, Mortensen PB, Sigurdsson E, Gustafsson O, Nyegaard M, Tuulio-Henriksson A, Ingason A, Hansen T, Suvisaari J, Lonnqvist J, Paunio T, Borglum AD, Hartmann A, Fink-Jensen A, Nordentoft M, Hougaard D, Norgaard-Pedersen B, Bottcher Y, Olesen J, Breuer R, Moller HJ, Giegling I, Rasmussen HB, Timm S, Mattheisen M, Bitter I, Rethelyi JM, Magnusdottir BB, Sigmundsson T, Olason P, Masson G, Gulcher JR, Haraldsson M, Fossdal R, Thorgeirsson TE, Thorsteinsdottir U, Ruggeri M, Tosato S, Franke B, Strengman E, Kiemeney LA, Melle I, Djurovic S, Abramova L, Kaleda V, Sanjuan J, de Frutos R, Bramon E, Vassos E, Fraser G, Ettinger U, Picchioni M, Walker N, Toulopoulou T, Need AC, Ge D, Yoon JL, Shianna KV, Freimer NB, Cantor RM, Murray R, Kong A, Golimbet V, Carracedo A, Arango C, Costas J, Jonsson EG, Terenius L, Agartz I, Petursson H, Nothen MM, Rietschel M, Matthews PM, Muglia P, Peltonen L, St Clair D, Goldstein DB, Stefansson K, Collier DA. Common variants conferring risk of schizophrenia. Nature. 2009;460(7256):744–747. doi: 10.1038/nature08186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrey EF, Bartko JJ, Lun ZR, Yolken RH. Antibodies to Toxoplasma gondii in patients with schizophrenia: a meta-analysis. Schizophr Bull. 2007;33(3):729–736. doi: 10.1093/schbul/sbl050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vacic V, McCarthy S, Malhotra D, Murray F, Chou HH, Peoples A, Makarov V, Yoon S, Bhandari A, Corominas R, Iakoucheva LM, Krastoshevsky O, Krause V, Larach-Walters V, Welsh DK, Craig D, Kelsoe JR, Gershon ES, Leal SM, Dell Aquila M, Morris DW, Gill M, Corvin A, Insel PA, McClellan J, King MC, Karayiorgou M, Levy DL, DeLisi LE, Sebat J. Duplications of the neuropeptide receptor gene VIPR2 confer significant risk for schizophrenia. Nature. 2011;471(7339):499–503. doi: 10.1038/nature09884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y, Someya T, Nawa H. Cytokine hypothesis of schizophrenia pathogenesis: evidence from human studies and animal models. Psychiatry Clin Neurosci. 2010;64(3):217–230. doi: 10.1111/j.1440-1819.2010.02094.x. [DOI] [PubMed] [Google Scholar]
- Xiao J, Jones-Brando L, Talbot CC, Jr, Yolken RH. Differential effects of three canonical Toxoplasma strains on gene expression in human neuroepithelial cells. Infect Immun. 2009;79(3):1363–1373. doi: 10.1128/IAI.00947-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yolken RH, Dickerson FB, Torrey EF. Toxoplasma and schizophrenia. Parasite Immunol. 2009;31(11):706–715. doi: 10.1111/j.1365-3024.2009.01131.x. [DOI] [PubMed] [Google Scholar]
- Yolken RH, Torrey EF. Are some cases of psychosis caused by microbial agents? A review of the evidence. Mol Psychiatry. 2008;13(5):470–479. doi: 10.1038/mp.2008.5. [DOI] [PubMed] [Google Scholar]