Abstract
Base-pair mismatches that occur during DNA replication or recombination can reduce genetic stability or conversely increase genetic diversity. The genetics and biophysical mechanism of mismatch repair (MMR) has been extensively studied since its discovery nearly 50 years ago. MMR is a strand-specific excision-resynthesis reaction that is initiated by MutS homolog (MSH) binding to the mismatched nucleotides. The MSH mismatch-binding signal is then transmitted to the immediate downstream MutL homolog (MLH/PMS) MMR components and ultimately to a distant strand scission site where excision begins. The mechanism of signal transmission has been controversial for decades. We have utilized single molecule Forster Resonance Energy Transfer (smFRET), Fluorescence Tracking (smFT) and Polarization Total Internal Reflection Fluorescence (smP-TIRF) to examine the interactions and dynamic behaviors of single Thermus aquaticus MutS (TaqMutS) particles on mismatched DNA. We determined that Taq-MutS forms an incipient clamp to search for a mismatch in ∼1 s intervals by 1-dimensional (1D) thermal fluctuation-driven rotational diffusion while in continuous contact with the helical duplex DNA. When MutS encounters a mismatch it lingers for ∼3 s to exchange bound ADP for ATP (ADP → ATP exchange). ATP binding by TaqMutS induces an extremely stable clamp conformation (∼10 min) that slides off the mismatch and moves along the adjacent duplex DNA driven simply by 1D thermal diffusion. The ATP-bound sliding clamps rotate freely while in discontinuous contact with the DNA. The visualization of a train of MSH proteins suggests that dissociation of ATP-bound sliding clamps from the mismatch permits multiple mismatch-dependent loading events. These direct observations have provided critical clues into understanding the molecular mechanism of MSH proteins during MMR.
Keywords: DNA mismatch repair, MutS, Single-molecule polarization, Single-molecule FRET, Single particle tracking, Diffusion mechanism
1. Introduction
DNA polymerases catalyze the pairing and covalent incorporation of complementary nucleotides into a transient single-stranded DNA (ssDNA) template within the replication fork [1]. The fidelity of this process is remarkable, resulting in less than one error per million nucleotides copied [2]. However, even that low error rate in human cells would lead to perhaps 1000 errors per cell division, which would ultimately have catastrophic consequence for genome integrity. Evelyn Witkin and Robin Holliday independently proposed the existence of mismatch repair (MMR) in 1964 to explain bromo-deoxyuracil incorporation in bacteria and gene conversion in fungi, respectively [3,4]. MMR primarily corrects polymerase misincorporation errors enhancing the overall fidelity of DNA replication up to 1000-fold. Loss of the MMR genes increases cellular mutation rates (Mutator or Mut) [5,6]. Defects in the human MMR machinery have been linked to the most common hereditary cancer predisposition syndrome hereditary non-polyposis colorectal cancer or Lynch syndrome (LS/HNPCC) as well as sporadic colorectal, endometrial, ovarian and upper urinary tract tumors [7]. The 20th anniversary of this connection was marked in 2013; a discovery that also established the concept that Mutators and genomic instability are a central cause of tumorigenesis [8].
The core components of MMR have been conserved throughout evolution [9]. MMR is an excision-resynthesis reaction that begins at a distant strand scission that may be located several thousand nucleotides either 3′ or 5′ from the mismatch. The excision tract extends uniquely from the strand scission to just past the mismatch suggesting a highly coordinated degradation process. MutS homologs (MSH) initiate MMR by recognizing mismatched nucleotides and then transmitting this discovery to downstream excision machinery (Fig. 1A and B). Bacterial MutS protein detects mismatches on DNA as a homodimer [10]. Eukaryotes appear to have duplicated and refined the MutS gene to form MSH heterodimers [11–13]. The MSH2-MSH6 heterodimer primarily recognizes base-base mispairs and single insertion/deletion unpaired nucleotides. In contrast, the MSH2-MSH3 heterodimer binds a few bases-base mispairs as well as unpaired nucleotides containing up to 8–12 insertion/deletion loop-type (IDL) mismatches [14–17].
Fig. 1.
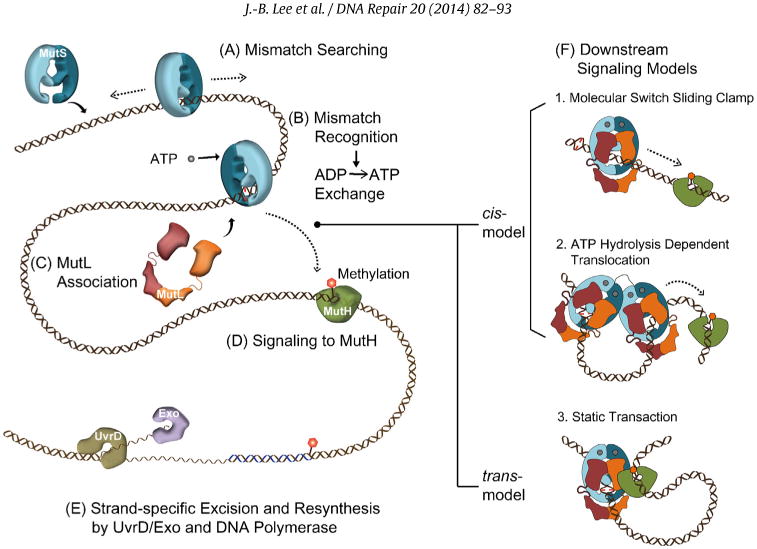
The mechanism of mismatch repair. (A) Mismatch searching performed by MutS homologs (MSH) occurs on duplex DNA. (B) Mismatch binding by an MSH provokes the exchange of bound ADP for ATP (ADP → ATP exchange). (C) The MutL homolog (MLH/PMS) associates with the MSH to transmit the mismatch binding signal. (D) In bacteria signal, the first downstream signaling component appears to be MutH that is responsible for introducing a strand scission on the newly synthesized unmethylated DNA strand. Methylation is controlled by the DNA adenine methylase (Dam) at GATC sequences. (E) Excision of the newly replicated strand is controlled by the UvrD helicase along with one of four exonucleases (see text) in concert with the MSH-MLH/PMS complex. Resynthesis of the single-stranded gap is performed by the replicative DNA polymerase. (F) Models for mismatch signal transmission: (1) Molecular Switch Sliding Clamp where ATP-bound MSH along with MLH/PMS moves by 1-dimensional thermal diffusion to downstream mismatch repair (MMR) machinery, (2) ATP-Hydrolysis Dependent Translocation where the MSH and MLH/PMS form a multimeric complex that translocates to the downstream machinery using the energy of ATP hydrolysis forming a bidirectional loop structure, and (3) Static Transactivation where an MSH-MLH/PMS complex is formed at the mismatch that then associates with downstream MMR machinery by 3D collision forming a static loop structure.
Structural analysis has revealed that MSH proteins are members of the AAA ATPase family [18,19]. MSH homodimer/heterodimer proteins appear to retain at least one ADP [20–22] that appears to control unregulated ATP hydrolysis [20]. Mismatch binding triggers the release of the bound ADP, allowing ATP binding by both subunits (ADP→ ATP exchange) [17,20,22–24] that results in dissociation from the mismatch. MutL homologs (MLH/PMS) are the first downstream MMR component that associates with ATP-bound MSH (Fig. 1C) [23,25]. While a GHKL (DNA Gyrase,Hsp90, Histidine Kinase and MutL) ATP binding and hydrolysis motif is a defining feature of MLH/PMS proteins [26,27], its role in MLH/PMS functions during MMR remains obscure. Bacterial MutL functions as a homodimer while eukaryotic MLH/PMS function as heterodimers. The major MLH/PMS in Sacchromyces cerevisiae (Sc) is ScMlh1-ScPms1 and in human hMLH1-hPMS2 (ScPms1 and hPMS2 are conserved homologs) [28–31].
The MMR excision tract occurs uniquely on the newly synthesized DNA strand. This strand specificity is an essential property of MMR that decreases the potential for mutations. In Escherichia coli (Ec), the MutS-MutL complex activates the EcMutH endonuclease that then introduces a single-strand scission on the unmethylated strand in a nearby hemimethylated GATC site (Fig. 1D). The DNA adenine methylase (Dam) symmetrically methylates GATC sequences throughout the genome [32], which then become transiently unmethylated on the newly replicated strand following DNA replication. The EcMutH strand scission serves as an entry site for the EcUvrD helicase and one of four exonucleases (Fig. 1E; 3′ → 5′ exonucleases, EcRecJ and EcExoVII; 5′ → 3′ exonucleases, EcExoI and EcExoX) [33] that remove the newly replicated strand to just pastthe mismatch [34–36]. The ability to perform Bidirectional excision from either the 3′ or 5′ direction toward the mismatch is a second defining property of MMR. The resulting DNA gap is resynthesized by the replication machinery and sealed by DNA ligase [36].
Outside of Gram-negative enteric bacteria such as E. coli, the mechanism of introducing strand specificity and bidirectionality is a mystery. An interesting hypothesis suggests that RNaseH introduces a strand specific scission in eukaryotes during removal of randomly incorporated ribonucleotides that have occurred during replication [37,38]. However, the mutator activity of the EcMutH strand-specific scission component in E. coli is nearly equivalent to EcMutS and EcMutL [5]. In contrast, the mutator activity of rnh (RNaseH) deletions is at least 100-fold less than ScMsh2 or ScPms1 deletions [15,38]. These observations suggest that components associated with eukaryotic strand-specific scission during MMR are at the very least redundant. A role of MSH proteins in directing strand specificity is unknown.
The mechanism of bidirectional excision in eukaryotes is also a puzzle since the 5′ → 3′ exonuclease, ExoI, has been the only exonuclease linked to eukaryotic MMR [39–41]. Interestingly, the 5′ → 3′ excision reaction does not require an MLH/PMS [42], while the 3′ → 5′ excision reaction requires MLH/PMS [43]. Several years ago the human hPMS2 protein was shown to contain a cryptic endonuclease activity [44]. Mutation of the conserved endonuclease domain of the ScPms1 or the Bacillus subtilis Gram-positive bacteria MutL (BsMutL) exhibited a mutator phenotype consistent with an MMR defect [45–47]. These observations suggested that the MLH/PMS endonuclease might create short DNA fragments during the 3′ → 5′ excision that might be presented to ExoI for 5′-degradation [44]. However, the low mutator phenotype of S. cerevisiae exoI mutations [48,49] might also suggest that the MLH/PMS endonuclease is redundant with ExoI. A direct role for MLH/PMS in strand excision might also incriminate MSH proteins in as yet unknown delivery roles associated with establishing bidirectionality.
2. The mechanics of mismatch repair
A major question associated with the MMR mechanism is how the mismatch recognition by MSH proteins is transmitted to the distant downstream site where strand excision is initiated. Three different models have been proposed that can be conceptually divided into cis or trans mechanisms along the mismatched DNA (Fig. 1F) [50]. The cis models include the Molecular Switch Sliding Clamp [23,24,51,52] and ATP Hydrolysis-Dependent Translocation [53,54] mechanisms (Fig. 1F). The Molecular Switch Sliding Clamp model proposes that ATP binding by MSH provokes a conformational transition that results in the formation of a sliding clamp that moves along the DNA to the strand scission site by 1Dimen-tional (1D) thermal diffusion (Fig. 1F) [23,24,51,52]. Once the MSH slides off the mismatch, additional MSH proteins may be loaded resulting in multiple bidirectional diffusing sliding clamps [55]. The ATP-bound sliding clamps attract MLH/PMS, and the complex activates strand excision in a dynamic process where redundant MSH-MLH/PMS complexes continuously turnover until the mismatch site is eliminated. In contrast, ATP hydrolysis drives the movement of the MSH-MLH/PMS complex from the mismatch to the target site in the ATP Hydrolysis-Dependent Translocation model (Fig. 1F) [53,54]. The Static Transactivation model proposes that the MutS (MSH) or a complex of MutS-MutL (MSH-MLH/PMS) remain associated with the mismatch and interact with the MutH (downstream excision activators) via 3D looping of the intervening DNA (Fig. 1F) [56]. This trans model has been largely dismissed since artificial blocks located on the DNA between the mismatch and the GATC site, inhibit MutH activation [57].
The crystal structures of TaqMutS, EcMutS and hMSH2-hMSH6 are remarkably similar, containing five identifiable subdomains (Domains I-V) that clasp the mismatched DNA [18,19,58] (Fig. 2A–C, insets). These domains involve mismatch binding (domain I) and ATPase activity (domain V) as well as connector domains (Domain II-IV). TaqMutS appears to interrogate the mismatched nucleotide (unpaired thymine, +dT; mispaired dT of the G/T mismatch for EcMutS and hMSH2-hMSH6) by stacking interactions with Phe39 (Phe36 in EcMutS and Phe432 in hMSH6). The interaction is further stabilized by hydrogen bonding with Glu41 (Glu38 in EcMutS and Glu434 in hMSH6) and DNA residues from the minor groove. This binding interface triggers a DNA bend toward to the major groove (Fig. 2A–C, insets).
Fig. 2.
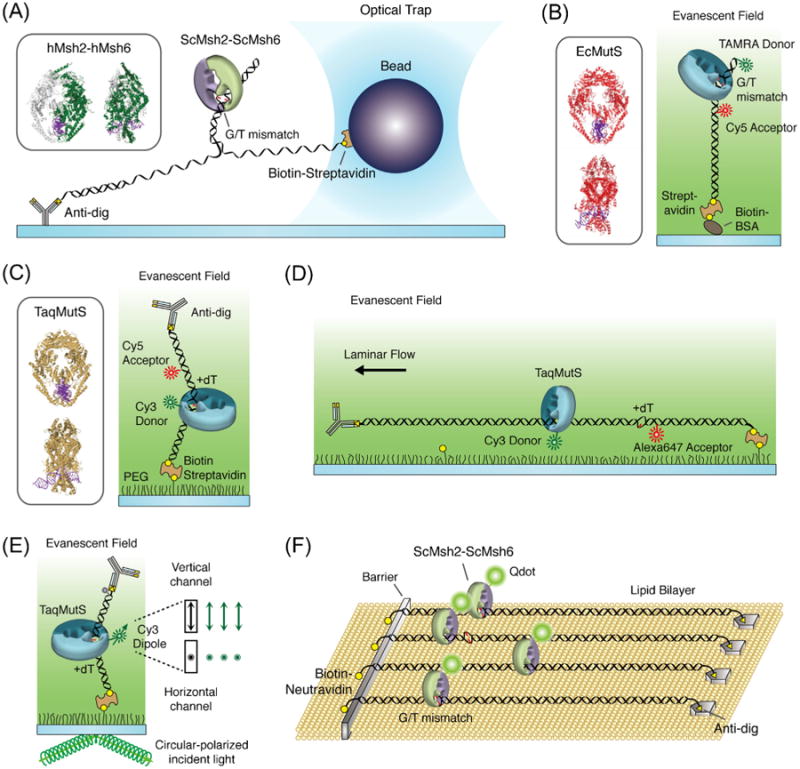
Single molecule methods for examining mismatch repair mechanics. (A) The optical trap system that examined ScMsh2-ScMsh6 on G/T mismatched DNA (inset: hMsh2-hMsh6 structure, PDB: 2O8F). (B) The smFRET system that examined DNA bending caused by EcMutS when it is bound to G/T mismatch by FRET between a TAMRA donor and a Cy5 acceptor located on either side of the mismatch (inset: EcMutS structure, PDB: 1E3M). (C) The smFRET system that monitored the interactions between donor Cy3-TaqMutS and a +dT mismatched DNA containing aCy5 acceptor located 9bp away from the mismatch (inset: TaqMutS structure, PDB: 1EWQ). (D) The combined single-particle tracking smFRET system that examined TaqMutS on +dT mismatched DNA. (E) The single-molecule polarization system that examined the rotational dynamics of TaqMutS on a +dT mismatched DNA. (F) The DNA curtain system containing three adjacent G/T mismatches DNA that examined ScMsh2-ScMsh6 and ScMlh1-ScPms1.
ATP-mediated conformational transitions from an “open” to a “closed” clamp were first visualized by electron microscopy with uranyl-acetate stained hMSH2-hMSH6 [52,59]. Additional DNA- and ATP-mediated conformational changes in the EcMutS and ScMsh2-ScMsh6 have been detailed by deuterium exchange mass spectrometry studies [60,61]. Interestingly, the structure of domains I and IV of TaqMutS appeared unresolved in the absence of DNA [19]. It is these domains along with domains II and III that ultimately form the clasp that encircles the bound mismatched DNA as visualized in the EM studies [18,19,52,58,59]. Until recently, the transition kinetics between these conformational states was largely unknown.
3. Diffusion mechanisms for protein DNA target location
Many proteins that interact with DNA exploit thermal-driven movement to search and locate specific target sites. Theoretical analysis clearly details the temporal enhancement of target location when 1D thermal diffusion along DNA is compared to random collision-dependent three-dimensional (3D) diffusion [62]. Two simple mechanisms for 1D diffusion have been described [63,64]: (1) “sliding” where the protein remains in continuous contact with the DNA, and (2) “hopping” where the protein is in discontinuous contact with the DNA. A hopping protein moving along DNA is proposed to transiently dissociate and then quickly re-associates with the DNA. Ultimately, hopping may result in complete dissociation from the DNA. The sliding and hopping 1D diffusion mechanisms may be distinguished by the effect of ionic strength on their respective diffusion coefficients [63,64]. The diffusion coefficient of a sliding protein should be unaffected by ionic strength, while a hopping protein is expected to exhibit faster diffusion at higher ionic strength.
The helical structure of the DNA may give rise to rotation for a sliding protein during a target search. The increased hydro-dynamic friction associated with rotation as well as encounters with both energy barriers and energy minima caused by DNA path heterogeneity will decrease the translational diffusion rate [65]. These dynamic issues together reduce the activation energy for each step during rotation-coupled translational diffusion. Rotation-coupled diffusion of a DNA binding protein can be theoretically assessed by determining whether the activation energy barrier (ΔG*), which may be estimated using the Arrhenius equation [klim = kobs exp(−ΔG*/kBT)], falls below the hypothetical energy landscape maximum for diffusion (∼2kBT) [66]. The random walk diffusion step rate (klim) may be obtained from the theoretical diffusion coefficient and the observed diffusion step rate (kobs) corresponds to an experimentally measured diffusion coefficient [67].
4. Single-molecule studies of MMR proteins: a historical overview
Single molecule analysis provides real-time and often visual details of biochemical reactions that cannot be garnered from bulk studies [68]. In 2005, the optical tweezers experiment was the first real-time single molecule study to be applied to MMR. This study measured the unzipping forces associated with ScMsh2-ScMsh6 binding to mismatched DNA [69] (Fig. 2A). The authors concluded that ScMsh2-ScMsh6 existed in two conformational modes, a high affinity mismatch binding mode and a sliding clamp mode as suggested by previous bulk studies [14,24,52]. To further investigate the behavior of ScMsh2-ScMsh6 in the presence of ATP, the authors measured the unzipping forces in the presence of the Lac repressor protein (LacI) bound to the Lac operator sequence (LacO) located 122 bp downstream from the G/T mismatch. The peak of the unzipping force between the mismatch and the LacI blockade suggested that ATP binding results in the release of ScMsh2-ScMsh6 from the mismatch. However, the majority of the events (∼90%) involved direct dissociation of ScMsh2-ScMsh6 or release of ScMsh2-ScMsh6 from the mismatch followed by direct dissociation. By comparison, similar real-time bulk study showed significantly stable ATP-bound ScMsh2-ScMsh6 sliding clamps on LacI/LacO blocked mismatched DNA [70], suggesting that the presence of an external force may limit an accurate kinetic analysis of various MSH-DNA interactions.
Two succeeding single molecule studies were reported in 2007 and 2010 that relied on the development of “DNA curtains” and fluorescent quantum dot (Qdot)-labeled MMR proteins. DNA curtains align hundreds of duplex λ DNAs along a nanofabricated barrier over a supported lipid bilayer within a Total Internal Reflection Fluorescence (TIRF) field [71,72]. The development of TIRF microscopy for single molecule analysis over the previous decade has allowed excitation of specific fluorophores that depend on the wavelength of the excitation laser, while simultaneously decreasing background fluorescence outside of the ∼100 nm thick TIRF field [73]. The large numbers of molecules in the TIRF field led to a determination of the diffusion characteristics of fluorescent Qdot-labeled ScMsh2-ScMsh6 on duplex DNA, even though these events are short lived and relatively rare [71]. The authors proposed that ScMsh2-ScMsh6 searched for mismatched nucleotides by 1D rotational diffusion along the DNA helix by theoretical comparisons of diffusion coefficients (see diffusion discussion above) [71]. Examination of the diffusion properties of ScMlh1-ScPms1 in the presence of ScMsh2-ScMsh6 as well as nucleosome DNA blocks suggested multiple diffusion mechanisms [72]. ScMsh2-ScMsh6 was unable to transit nucleosome blocks while diffusing on duplex DNA. In contrast, ScMlh1-ScPms1 appeared to easily transit nucleosomes but often appeared to associate with ScMsh2-ScMsh6 bound to the same duplex DNA. Unfortunately, neither of these DNA curtain studies examined DNA containing a mismatch, leaving important kinetic transitions in the real-time observations of MMR unresolved.
Our group examined the single-stranded DNA (ssDNA) binding characteristics of EcMutL in 2010 using both single molecule Förster Resonance Energy Transfer (FRET) and a flow-stretching system [74]. The FRET system utilizes a fluorescent donor dye (Cy3) placed at the junction of a short duplex DNA attached to a passivated surface, and a fluorescent acceptor dye (Cy5) on the end of a 3′-ssDNA tail. In our studies, EcMutL interaction with the 3′-tailed ssDNA substrate decreased FRET, suggesting an increase in the distance between the Cy3 and Cy5 fluorophores [74]. The interaction was dependent on the ssDNA tail and ATP. Attaching a long λ DNA-based 5.3 kb ssDNA to a passivated surface on one end and a 2.8 μm magnetic bead at the other end within a defined laminar flow visualized real-time EcMutL binding under a fixed hydrodynamic force. These studies confirmed that multiple EcMutL proteins may bind ssDNA [25,75]. However, the binding activity decreased to zero at physiological ionic strength (120–150 mM), suggesting additional complexity in MLH/PMS–ssDNA interactions.
A FRET single molecule system was also developed in 2010 to examine EcMutS binding to mismatched DNA [76]. The DNA substrate contained TAMRA and Cy5 fluorophores 10bp and 8bp on either side of a G/T mismatch (Fig. 2B). Binding of EcMutS to the mismatch resulted in a DNA bend that shortened the distance between the fluorophores, which increased the FRET. While the data appeared to suggest a simple on–off binding kinetics, the authors projected multiple embedded conformational states based on transition analysis combined with kinetic lifetime examination (TACKLE). These considerations appeared to suggest several other DNA bending states in addition to that observed in the static crystal structures.
5. The first real-time images of single MMR proteins on mismatched DNA
While discussed in more detail below, our group reported the real-time imaging of TaqMutS on single DNA molecules containing a mismatch in 2011 and 2012 [77,78]. A FRET system was developed that monitored the interaction of Cy3-labeled TaqMutS with a Cy5-labled 74-mer DNA containing a mismatch (Fig. 2C). The DNA substrate was attached to the surface via biotin–streptavidin and the remaining free-end blocked with an anti-digoxigenin antibody bound to a 5′-digoxigenin. Mismatch binding by Cy3-TaqMutS corresponded to a relatively high FRET efficiency (E ∼ 0.8) [77]. Remarkably, a lower FRET efficiency (E ∼ 0.5) was observed for the low frequency binding events to duplex DNA in the absence of a mismatch as well as TaqMutS bound to DNA containing a mismatch in the presence of ATP. Both of these lower FRET binding events were subsequently determined to be time-averaged FRET of TaqMutS particles transiting the entire length of the short oligonucleotide during the 30 ms acquisition period. The difference was that the lifetime of the duplex DNA binding events was ∼1 s at physiological ionic strength, while the lifetime of the TaqMutS associated with the mismatched DNA in the presence of ATP was ∼10 min over a wide range of ionic strengths [77]. Another group later examined the conformational transitions of TaqMutS bound to mismatched DNA in 2012 using FRET between Cy3 and Cy5 bound to individual domain I monomers within the dimer [79]. Taken together, these observations provided evidence that TaqMutS transited between at least three clamped conformations on the DNA: a mismatch searching clamp, a mismatch binding clamp and an ATP-bound sliding clamp.
The spatial positioning of MutS on mismatched DNA cannot be determined using short oligonucleotide DNAs. This limitation inspired the construction of a 15.3 kb DNA that contained a single mismatch (+dT) as well as an adjacent Alexa647 fluorophore that could act as a FRET acceptor with Cy3-labeled TaqMutS (Fig. 2D). One end of the 15.3 kb DNA was immobilized with 5′-biotin bound to a streptavidin-coated surface and the DNA was then stretched with hydrodynamic force by applying a laminar flow. The combination of single-particle tracking and single-molecule FRET was then used to visualize the diffusion dynamics of single and/or multiple TaqMutS on the mismatched DNA. Parallel studies examined the rotational dynamics of the searching clamp and ATP-bound sliding clamp by measuring changes in perpendicular and parallel polarization components (relative to the microscope stage) of Cy3-labeled TaqMutS (Fig. 2E) [78]. By determining the polarization of Cy3-labeled TaqMutS on DNAs that approached the protein footprint, these studies experimentally verified that TaqMutS searched for a mismatch by rotational diffusion along the helix while in continuous contact with the DNA. In contrast, once ATP-bound TaqMutS sliding clamps dissociate from a mismatch they rotate freely while in discontinuous contact with the DNA. Details of these single-molecule studies will be discussed through the following sections.
The dynamics of ScMsh2-ScMsh6 on λ DNA curtains containing 3 neighboring mismatches was reported in 2012 (Fig. 2F) [80]. They confirmed that the dynamics of these eukaryotic MSH proteins were largely identical to TaqMutS. Furthermore, they also showed that the presence of ScMlh1-ScPms1 does not change mismatch searching and ATP-dependent transitions of ScMsh2-ScMsh6 [80].
6. Single molecule FRET studies of TaqMutS on DNA: the details
TaqMutS contains a single cysteine residue at position 42 (C42) in domain I (mismatch interrogation domain). Unfortunately, maleimide chemistry conjugation of a fluorophore to TaqMutS (C42) completely inactivated both mismatch binding and mismatch-dependent ATPase activity. Importantly, a TaqMutS (C42A) substitution mutation had no effect on activity. This observation allowed the construction of a TaqMutS (C42A, T469C), where the TaqMutS (T469) residue is located in domain IV far from critical mismatch interrogation and ATP processing domains. Moreover, when the TaqMutS (C42A, T469C) was conjugated with a Cy3 at residue 469 (Cy3-TaqMutS) there was no discernable effect on any MMR activities. To detect FRET using the Cy3-TaqMutS donor, a Cy5 acceptor was placed at an identical position on a 74-mer duplex DNA or a DNA containing an unpaired +dT mismatch at position 38 from a 5′-biotin. The Cy5 was located at position 47 that is 9 bp away from 3′ of the mismatch (Fig. 3A). These DNA substrates were independently immobilized on a quartz surface using biotin–streptavidin and the opposing 5′-end was blocked with digoxigenin–antidigoxigenin antibody to prevent any potential end-binding and/or end-dissociation during the analysis (Fig. 3A).
Fig. 3.
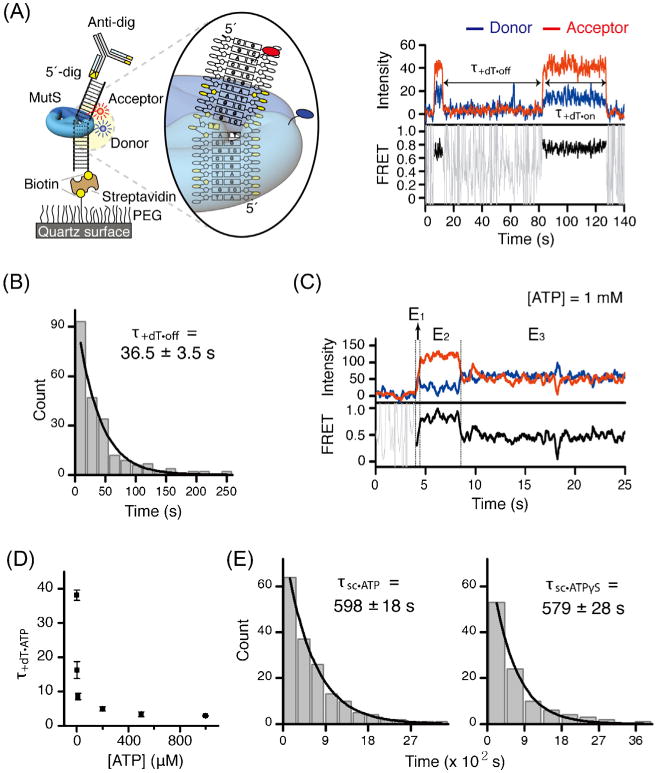
TaqMutS forms a remarkably stable clamp on mismatched DNA. (A) Left: the schematic representation of the smFRET system that directly monitors the interaction between Cy3-TaqMutS (donor) and a Cy5-labeled (acceptor) DNA containing a +dT mismatch (see Fig. 2C). Right: a representative time trace of donor-acceptor intensity and the resulting FRET efficiency. (B) The lifetime of TaqMutS on +dT mismatch in the absence of ATP. (C) A representative time trace in the presence of ATP (1 mM) showing three FRET transitions: E1 searching TaqMutS, E2 mismatch binding TaqMutS, and E3 ATP-bound sliding clamp TaqMutS. (D) The lifetime of TaqMutS on the +dT mismatch at various ATP concentrations. (E) The lifetime of ATP-bound TaqMutS (598 s) and ATPγS-bound TaqMutS (579 s) sliding clamps.
The first intriguing result was that the dwell time and ultimately the binding constant (Kd) of Cy3-TaqMutS was dramatically decreased by blocking the open end of the DNA with digoxigenin–antidigoxigenin [77]. These observations suggested that that mismatch searching TaqMutS forms an incipient clamp on duplex DNA as projected from deuterium exchange mass spectrometry studies of ScMsh2-ScMsh6 [60]. TaqMutS bound to duplex DNA displayed a FRET efficiency of 0.48 ± 0.14 (mean ± s.d.) [77]. Importantly, the FRET efficiency was found to decrease when the length of the duplex DNA was increased to 100 bp. Taken together, these results were consistent with the notion that the incipient TaqMutS clamps were transiting the entire length of the duplex DNA numerous times during the 30 ms acquisition period, and that the FRET efficiency was time-averaged over many positions along the DNA [77].
When Cy3-TaqMutS binds to the unpaired +dT, the resulting distance between Cy3 and Cy5 is estimated to be 4–5 nm, which corresponds to a calculated FRET efficiency of 0.75 assuming a Föster radius of 5.5 nm. As expected, in the absence of ATP the Cy5 signal abruptly increases to a FRET efficiency of 0.78 ± 0.16 (mean ± s.d.) (Fig. 3A). The kinetic increases and decreases in FRET efficiency suggested a simple two-state binding (on–off) with corresponding dwell times (τ+dT·on and τ+dT·off). Fitting a single exponential decay to τ+dT·on from several hundred observations gave a mean population dwell time of 36.2 ± 3.5 s (mean ± s.e.) (Fig. 3B). Examining the τ+dT·off over several concentrations of TaqMutS and combining that data with the concentration-independent τ+dT·on resulted in a calculated Kd = 16.2 ± 2.1 nm (mean ± s.e.), which agreed well with other single molecule [81] and bulk measures [82,83].
Fluorescence intensity and FRET traces of TaqMutS binding to mismatched DNA in the presence of ATP consistently showed a short intermediate FRET state (E1 =0.48 ± 0.12, mean ± s.d.), followed by a high FRET state (E2 = 0.78 ± 0.15, mean ± s.d.), followed by an intermediate FRET state (E3 = 0.49 ± 0.17, mean ± s.d.) (Fig. 3C). The dwell time of the E1 intermediate FRET state (τon·ATP) was often shorter than our time resolution (30 ms), while the dwell time of the high FRET state (τ+dT·ATP) was inversely proportional to the ATP concentration which asymptotically approached ∼3 s (Fig. 3D). These latter observations imply an ATP-dependent conformational transition from the E2 high FRET state to the E3 intermediate FRET state. Remarkably, the mean dwell time of the E3 intermediate FRET state was τSC·ATP = 598 ± 18 s (Fig. 3E, left). Moreover, the mean dwell time of the E3 intermediate FRET state did not change when ATP was substituted with the poorly hydrolysable analog ATPγS (τSC·ATPγS = 579 ± 28 s) (Fig. 3E, right). The result indicates that the transition time from the high FRET state as well as the stability of the intermediate FRET state on the mismatched DNA does not require ATP hydrolysis. Together with previous bulk biochemical data [23,24,52,84], these data strongly supported the conclusion that the E1, E2, and E3 FRET states corresponded to mismatch-searching TaqMutS, mismatch-bound TaqMutS and ATP-bound TaqMutS sliding clamp, respectively [77]. Importantly, this high-resolution single molecule FRET analysis provided the first kinetic details of the TaqMutS conformational transitions from mismatch searching, to mismatch binding, to ATP-bound sliding clamp. Because both the E1 and E3 intermediate FRET states represented time-averaged movement along the entire length of the oligonucleotide DNA, these studies also suggested the existence of two dynamically different forms of TaqMutS that rely on thermal fluctuation-driven diffusion.
7. Two distinct diffusion mechanisms of TaqMutS
Real-time single particle tracking studies have directly visualized facilitated target location by various DNA binding proteins as well as distinct diffusion mechanisms [67,71,77,85–88]. However, the detailed diffusion mechanics of MSH proteins on DNA containing a mismatch were unknown.
To examine real time diffusion of TaqMutS clamps on mismatched DNA a 15.3 kb DNA was constructed from a λ-phage DNA that contained both a single +dT and a single Alexa647 fluorophore with similar FRET characteristics to Cy5 as an acceptor (Fig. 4A). λ DNA was digested with BsrGI or ApaI, which results in 5204bp (5.2kb) and 10,076bp (10.1 kb) fragments that contain a 4 nt single-stranded DNA (ssDNA) 5′-overhang and a 12 nt ssDNA left cohesive end (cosL). Alexa647 was covalently conjugated to an amine-modified dT nucleotide in a 47 mer. The cosL tail of the 5.2 kb fragment was annealed and ligated with the Alexa647-ssDNA oligonucleotide, and the BsrGI ssDNA tail was annealed and ligated with a 23-mer oligonucleotide containing a 5′-biotin. The cosL tail of 10.1 kb fragment was annealed and ligated with a 48-mer ssDNA oligonucleotide that was complementary to the Alexa647 DNA but additionally contained a +dT, while the ApaI ssDNA tail was annealed and ligated with a 29-mer oligonucleotide containing a 5′-digoxigenin. A single +dT mismatch with an Alexa647 fluorophore located 9 bp away was formed by annealing the Alexa647-containing 10.1 kb DNA overhang with its +dT-containing 5.2 kb DNA complement. The resulting 15.3 kb DNA was tethered to a passivated surface with biotin–streptavidin and steadily stretched by applying hydrodynamic drag to the DNA in a laminar flow of a reaction buffer.
Fig. 4.
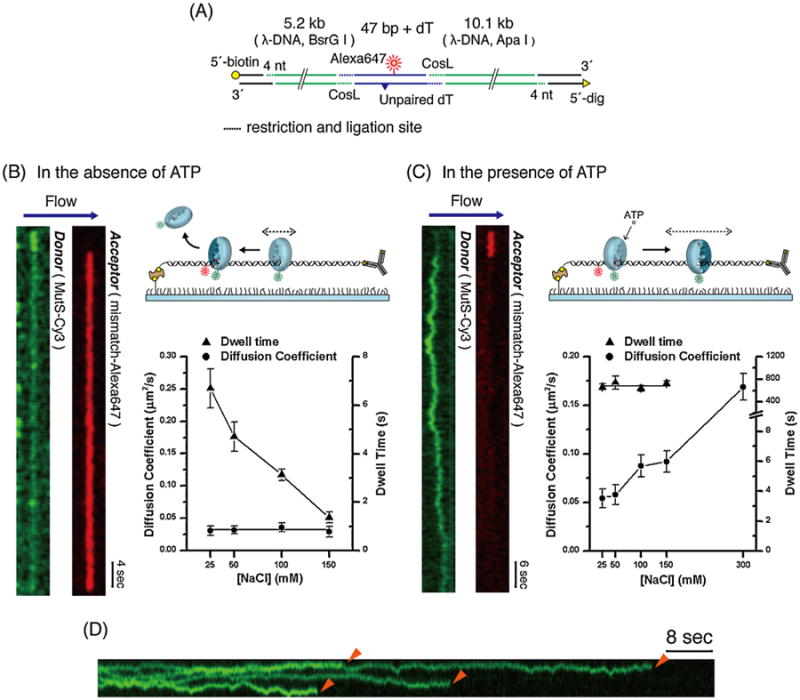
Two different diffusion mechanics of TaqMutS during MMR. (A) Illustration of a 15.3 kb long DNA that contains an acceptor Alexa647 and +dT mismatch (see Fig. 2E). (B) Left: kymogram of the spatial positions of Cy3-TaqMutS donor and mismatch-associated acceptor (FRET) signals over time in the absence of ATP. Right: the diffusion coefficient and the lifetime of mismatch searching TaqMutS at various salt (NaCl) concentrations. (C) Left: kymogram of the spatial positions of Cy3-TaqMutS donor and mismatch-associated acceptor (FRET) signals overtime in the presence of ATP. Right: the diffusion coefficient and the lifetime of ATP-bound TaqMutS sliding clamp at various salt (NaCl) concentrations. (D) kymogram tracking multiple TaqMutS particles on a single 15.3 kb DNA containing a mismatch.
Cy3-TaqMutS was visualized on the flow-stretched DNA containing a mismatch under a wide-field prism-type TIRF microscope. Fig. 4B (left) displays a typical kymogram showing the time-dependent binding (read top-to-bottom) of Cy3-TaqMutS to the 15.3 kb mismatched DNA in the absence of ATP. One observes the Cy3-TaqMutS donor (green) binding to the DNA and searching for a mismatch. When the Cy3-TaqMutS encounters the mismatch the Cy3 emission decreases while the Cy5 FRET acceptor (red) emission simultaneously appears (E ∼ 0.7). This FRET emission signifying Cy3-TaqMutS bound to the mismatch remains for ∼50s before abruptly disappearing.
Prior to mismatch binding, the TaqMutS is non-specifically bound and appears to move along the DNA by 1D-diffusion. By tracking multiple molecules on both the mismatch-containing and duplex DNA, we could determine the dependence of the dwell-time and the diffusion coefficient on ionic strength (Fig. 4B, right) [77]. We found that the diffusion coefficient appeared constant over a wide range of ionic conditions [77]. These observations imply that mismatch searching TaqMutS maintains continuous contact while sliding along the DNA during thermal-driven diffusion. Remarkably, the dwell-time of searching TaqMutS decreased with increasing ionic strength suggesting clear ionic protein-DNA interactions. At physiological ionic conditions (130–150mM) the well time was ∼1 s, suggesting that TaqMutS was capable of searching ∼700bp of naked DNA during each encounter. Interestingly, there appeared to be a few direct 3D diffusion-driven binding events to the mismatch for both TaqMutS and ScMsh2-ScMsh6 [77,80]. However, to absolutely distinguishing such 3D events from short 1D diffusion encounters, enhanced spacial resolution will be required.
In the presence of ATP the pattern of Cy3-TaqMutS on the 15.3 kb DNA containing a mismatch is considerably different (Fig. 4C, left). In a typical kymogram one observes a period of Cy5 emission indicative of a high FRET interaction (E ∼ 0.7) between Cy3-TaqMutS and the mismatch. That is followed by a long period of Cy3 emission in which the Cy3-TaqMutS randomly moves along the duplex DNA adjacent to the mismatch; in most cases until the fluorophore signal is lost as a result of photobleaching. This pattern is consistent with TaqMutS interacting with a mismatch followed by the formation of an ATP-bound sliding clamp. Time-lapse analysis suggests that the diffusion coefficient of ATP-bound Cy3-TaqMutS increases with increasing ionic strength (Fig. 4C, right). This observation is consistent with a hopping mechanism for thermal diffusion where TaqMutS is in discontinuous contact with the DNA. Moreover, the dwell-time appears to be constant (τSC·ATP ∼ 600 s) over a wide range of ionic strength including physiological ionic strength (Fig. 4C, right). These results suggest that ATP-bound TaqMutS sliding clamps may traverse 10's of thousands of bp of naked DNA by 1D diffusion and exist for significant periods of time to displace intervening DNA binding proteins [89,90].
The long dwell-times of ATP-bound TaqMutS sliding clamps suggested that we might be capable of imaging multiple sliding clamps. Indeed, we observed many such events (Fig. 4D) and found that the number of molecules containing multiple sliding clamps increased with protein concentration as expected [78]. These images are most consistent with the Molecular Switch Sliding Clamp model for MMR [23,24,51,52].
8. Rotational dynamics of TaqMutS
Both ScMsh2-ScMsh6 and TaqMutS have been proposed to follow the helical contour of the DNA by 1D rotational diffusion while searching for a mismatch [77,80]. These conclusions were based on a comparison of theoretical diffusion coefficients with and without rotational diffusion to the observed diffusion coefficient. To experimentally resolve the rotational dynamics of TaqMutS on both duplex and mismatched DNA, a single molecule polarization decay technique was developed [78]. The fluorescent emissions of Cy3-TaqMutS excited by a circularly polarized incident light were separately collected for the components parallel (I∥) and perpendicular (I⊥) to the plane of the microscope stage. The polarization (P) was calculated as the ratio of (I∥ − I⊥)/(I∥ +I⊥) for the anisotropic extents of the emission signal. This technique is only viable if the rotational depolarization of the fluorophore is significantly slower than the acquisition time [91–93]. To determine this, we immobilized Cy3-TaqMutS to the surface and examined the polarization of a population of molecules. We found that the polarization of these randomly oriented Cy3-TaqMutS was broadly distributed around zero [78]. These observations suggested that the rotational freedom of the Cy3 fluorophore was significantly constrained and that the polarization dynamics were largely dependent on the orientation of the TaqMutS protein.
With this system in place, representative traces and the polarization distribution of mismatch searching TaqMutS (Fig. 5A), mismatch binding TaqMutS (Fig. 5B) and the ATP-bound TaqMutS sliding clamp (Fig. 5C) were examined. It is clear that the polarization of both searching TaqMutS and ATP-bound TaqMutS sliding clamps had decayed to near zero, while the polarization of mismatch bound TaqMutS remained broadly distributed. These results suggested that searching TaqMutS and ATP-bound TaqMutS sliding clamps had enjoyed significant rotational freedom, while the mismatch bound TaqMutS remained randomly oriented and rotationally constrained [78]. Forthe mismatch-searching TaqMutS this process is easy to visualize. Based on a diffusion coefficient of 0.032 μm2/s, it takes 0.18 ms for mismatch-searching TaqMutS to travel 10.5 bp (one helical pitch), which corresponds to the period of the Cy3-TaqMutS dipole rotation. Thus, mismatch-searching TaqMutS can make 167 full rotations around the helical axis of DNA within our time resolution of 30 ms, which results in the nearly identical I∥ and I⊥. These observations are consistent with the conclusion that the depolarization of Cy3-TaqMutS represents a time-averaged polarization during rotational diffusion along the effective DNA length. A similar helical rotation argument can be made for the ATP-bound TaqMutS sliding clamps. However, the “hopping” diffusion dynamics determined with single particle tracking seemed inconsistent with continuous helical rotation of ATP-bound TaqMutS sliding clamps.
FIg. 5.
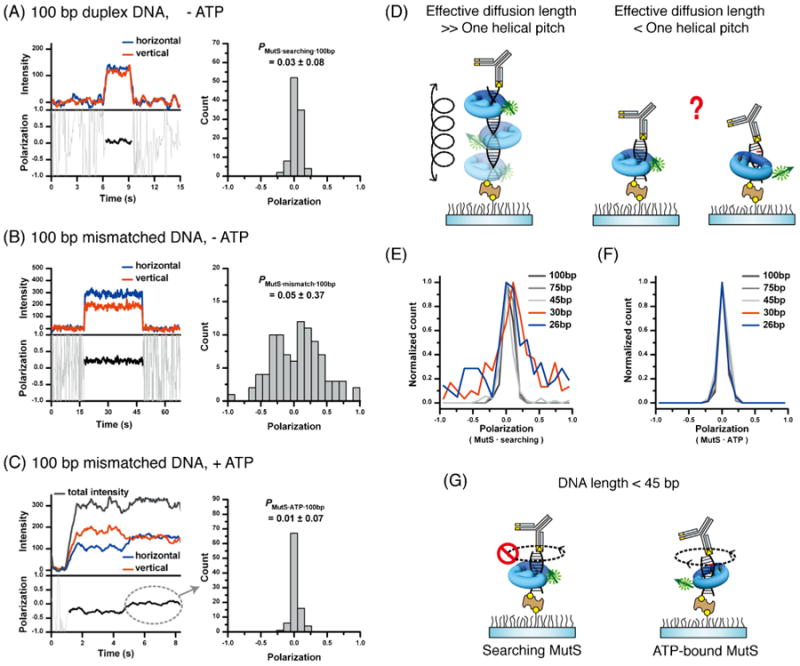
The rotational dynamics of TaqMutS. The polarization of Cy3-TaqMutS was determined on single DNA molecules (see Fig. 2E). (A) Left: representative time trace of Cy3-TaqMutS on a 100bp duplex DNA in the absence of ATP. Right: population histogram of Cy3-TaqMutS polarization on a 100bp duplex DNA in the absence of ATP. (B) Left: representative time trace of Cy3-TaqMutS on a 100bp DNA containing a +dT mismatch in the absence of ATP. Right: population histogram of Cy3-TaqMutS polarization on a 100bp DNA containing a +dT mismatch in the absence of ATP. (C) Left: representative time trace of Cy3-TaqMutS on a 100 bp DNA containing a +dT mismatch in the presence of ATP. Right: population histogram of Cy3-TaqMutS polarization on a 100 bp DNA containing a +dT mismatch in the presence of ATP. Note that only polarization following sliding clamp formation was plotted (see dashed circle). (D) Illustration of polarization changes associated with rotation of Cy3-TaqMutS on a DNA with a diffusion length greater than one helical pitch (left) or a diffusion length less than one helical pitch (right). Note that the footprint of TaqMutS is 24 bp [112]. Thus, a DNA substrate in which TaqMutS contacts less than one helical pitch must at least accommodate the footprint of the protein. (E) The normalized count of polarized Cy3-TaqMutS on various lengths of duplex DNA. (F) The normalized count of polarized Cy3-TaqMutS on various lengths of DNA containing a +dT mismatch in the presence of ATP. (G) Illustration of the lack of rotational diffusion of TaqMutS on a duplex DNA shorter than 45 bp, and the free rotational diffusion of ATP-bound TaqMutS sliding clamps on DNA containing a +dT mismatch in the presence of ATP.
To further resolve the helical rotational dynamics we realized that a searching TaqMutS that is in continuous contact with the DNA backbone would be rotationally constrained as the DNA length approached the binding footprint (Fig. 5D). In this limit case the polarization should remain broadly distributed similar to mismatch-bound TaqMutS. We examined the polarization distribution of searching TaqMutS on duplex DNA from 26 to 100bp in length (Fig. 5E). The results suggest that the polarization distribution of searching TaqMutS is broadly distributed at DNA lengths that approach the footprint, but decays to time-averaged near zero polarization when the DNA length and potential for rotational diffusion are increased [78]. Taken together, these studies are consistent with the conclusion that TaqMutS searches for a mismatch by rotational diffusion while in continuous contact with the DNA helix [78].
We performed a similar experiment with Cy3-TaqMutS in the presence of ATP on DNA containing a mismatch (Fig. 5F). The results suggest that a near zero polarization at all DNA lengths [78]. These observations are consistent with the conclusion that ATP-bound TaqMutS sliding clamps rotate freely while diffusing in discontinuous contact with the DNA (Fig. 5G) [78]. The results suggest an ATP-driven switch in the DNA diffusion dynamics of MutS protein [78].
9. Summary
A variety of single-molecule studies have successfully explored the biochemical and biophysical problems associated with MMR. The initiation of MMR by MSH proteins appears to be largely resolved (Fig. 6). The mismatch searching MSH tracks along the relatively smooth helical DNA backbone by 1D rotational diffusion [78]. An encounter with a mismatch stalls the 1D diffusion and allows the MSH protein to exchange ADP → ATP [24]. It is likely that diffusion stalling occurs because the MSH transitions from a smooth helical backbone to a flexible DNA structure that surrounds the mismatch [84]. The recognition of a flexible DNA structure rather than the actual mismatched nucleotides [94] or base-stacking alterations [95] may explain the wide-range of mismatches, lesions and DNA structures that are recognized by MSH proteins [84].
Fig. 6.
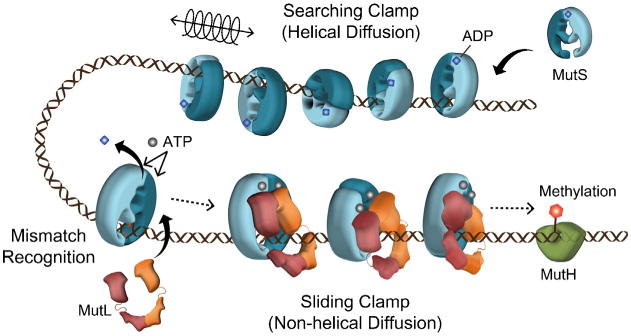
Summary of the molecular mechanism of MMR. See text for description.
The conformational transition of mismatch searching MSH to ATP-bound MSH sliding clamps alters the fundamental diffusion characteristics of the MSH protein [78]. The unconstrained movement of the ATP-bound MSH sliding clamp increases the degrees of freedom for interactions with the downstream proteins as well as a faster transition to the strand scission site where MMR excision is initiated (4-fold faster than searching MutS). The direct real-time imaging of MutS and MSH proteins on mismatched DNA uniquely supports the Molecular Switch Model for MMR although the complete MMR system has not been reconstituted on a single molecule system. Importantly, real-time tracking of both ScMsh2-ScMsh6 and ScMlh1-ScPms1 appears to confirm the complex moves away from the mismatch presumably toward the downstream strand excision components such as MutH in E. coli (Fig. 6).
10. Perspectives
Single molecule analysis of both biochemical and cellular reaction is rapidly expanding largely because of its capability for imaging molecules in real-time, observing intermediate and rare populations, fast acquisition times (usually in the low ms range), and single particle tracking with nm resolution. Clearly, there are a number of single molecule applications that will facilitate solution of the remaining biophysical and genetic problems in MMR.
An ultimate goal in single molecule MMR studies is to image the entire reaction. However, a number of partial reactions remain uncertain. Genetic and bulk biochemical data that suggest the yeast and human MSH heterodimers require ATP binding by both subunits to form a sliding clamp [20,22], while conflicting biochemical data with bacterial and human MSH suggest alternating or mixed adenosine nucleotide binding [21,96,97]. Because Cy3 and Cy5 derivatives if ADP and ATP exist, a single molecule fluorescent tracking of FRET study would seem plausible.
The biophysical role of PCNA (β-clamp in bacteria) in MMR is largely unknown. There appear to be significant interactions between MMR components and PCNA/β-clamp [98-102], genetic mutations that affect PCNA interaction with MMR components display elevated mutation rates [98], and PCNA and its clamp-loader RFC are absolutely required for 3′ MMR [43]. The development of specific fluorophore labeling systems for MMR components and PCNA/β-clamp could be used in both single molecule fluorescent tracking and FRET studies to probe their detailed roles in 3′ MMR.
While much is known about the mismatch initiation events associated with MSH and MLH/PMS, virtually nothing is know about the mechanism of strand excision and removal of the mismatch strand. The single molecule flow-stretching analysis [103] would appear to be a useful technique to examine the kinetics of MSH-EXOI activities in 5′ MMR.
Recently, ScMSh2-ScMsh6/ScMlh1-ScPms1 and EcMutS/EcMutL were visualized in living yeast and bacterial cells, respectively as fluorescent fusion proteins [104,105]. However, these studies did not contain high enough resolution to image individual proteins. Single molecule analysis of DNA replication and transcription has been performed [106,107]. These studies suggest an additional avenue for MMR studies will almost certainly entail single molecule analysis in living cells.
Ultimately, one of the major issues in single molecule analysis is molecular resolution. Microscopic imaging is by nature restricted by the diffraction limit (several 100 nm). Even large particles such as MSH proteins have a molecular size in the 10 nm range. A number of super resolution methods have been developed to localize particles [108–110]. In addition, polarization-controlled photoswitching appears to be a simple and non-stochastic super-resolution imaging process [111]. The development and application of low nanometer scale super resolution to real-time single molecule studies will almost certainly shift the paradigms of traditional biochemical and genetic analysis.
Supplementary Material
Acknowledgments
This work was supported by the National Research Foundation (NRF) of Korea and the Ministry of Education, Science, and Technology (MEST; 2011-0013901 and 2010-0019706; J-BL) and NIH grant CA67007 (RF).
Footnotes
Conflict of interest: None.
Appendix A. Supplementary data: Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.dnarep.2014.02.014.
Contributor Information
Jong-Bong Lee, Email: jblee@postech.ac.kr.
Richard Fishel, Email: rfishel@osu.edu.
References
- 1.Friedberg EC, Walker GC, Siede W, Wood RD, Schultz RA, Ellenberger T. DNA Repair and Mutagenesis. 2nd. American Society of Microbiology; Washington, DC: 2006. [Google Scholar]
- 2.Kunkel TA. DNA replication fidelity. J Biol Chem. 2004;279:16895–16898. doi: 10.1074/jbc.R400006200. [DOI] [PubMed] [Google Scholar]
- 3.Witkin EM. Pure clones of lactose negative mutants obtained in Escherichia coli after treatment with 5-bromouracil. J Mol Biol. 1964;8:610–613. doi: 10.1016/s0022-2836(64)80017-6. [DOI] [PubMed] [Google Scholar]
- 4.Holliday RA. A mechanism for gene conversion in fungi. Genet Res. 1964;5:282–304. doi: 10.1017/S0016672308009476. [DOI] [PubMed] [Google Scholar]
- 5.Cox EC. Bacterial mutator genes and the control of spontaneous mutation. Ann Rev Genet. 1976;10:135–156. doi: 10.1146/annurev.ge.10.120176.001031. [DOI] [PubMed] [Google Scholar]
- 6.Loeb LA. Mutator phenotype may be required for multistage carcinogenesis. Cancer Res. 1991;51:3075–3079. [PubMed] [Google Scholar]
- 7.Martin-Lopez JV, Fishel R. The mechanism of mismatch repair and the functional analysis of mismatch repair defects in Lynch syndrome. Fam Cancer. 2013;12:159–168. doi: 10.1007/s10689-013-9635-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fishel R, Kolodner RD. Identification of mismatch repair genes and their role in the development of cancer. Curr Opin Genet Dev. 1995;5:382–395. doi: 10.1016/0959-437x(95)80055-7. [DOI] [PubMed] [Google Scholar]
- 9.Modrich P, Lahue R. Mismatch repair in replication fidelity, genetic recombination, and cancer biology. Annu Rev Biochem. 1996;65:101–133. doi: 10.1146/annurev.bi.65.070196.000533. [DOI] [PubMed] [Google Scholar]
- 10.Su SS, Modrich P. Escherichia coli mutS-encoded protein binds to mismatched DNA base pairs. Proc Natl Acad Sci U S A. 1986;83:5057–5061. doi: 10.1073/pnas.83.14.5057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Acharya S, Wilson T, Gradia S, Kane MF, Guerrette S, Marsischky GT, Kolodner R, Fishel R. hMSH2 forms specific mispair-binding complexes with hMSH3 and hMSH6. Proc Natl Acad Sci U S A. 1996;93:13629–13634. doi: 10.1073/pnas.93.24.13629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bocker T, Barusevicius A, Snowden T, Rasio D, Guerrette S, Robbins D, Schmidt C, Burczak J, Croce CM, Copeland T, Kovatich AJ, Fishel R. hMSH5: a human MutS homologue that forms a novel heterodimer with hMSH4 and is expressed during spermatogenesis. Cancer Res. 1999;59:816–822. [PubMed] [Google Scholar]
- 13.Fishel R, Wilson T. MutS homologs in mammalian cells. Curr Opin Genet Dev. 1997;7:105–113. doi: 10.1016/s0959-437x(97)80117-7. [DOI] [PubMed] [Google Scholar]
- 14.Gradia S, Acharya S, Fishel R. The role of mismatched nucleotides in activating the hMSH2-hMSH6 molecular switch. J Biol Chem. 2000;275:3922–3930. doi: 10.1074/jbc.275.6.3922. [DOI] [PubMed] [Google Scholar]
- 15.Harrington JM, Kolodner RD. Saccharomyces cerevisiae Msh2–Msh3 acts in repair of base–base mispairs. Mol Cell Biol. 2007;27:6546–6554. doi: 10.1128/MCB.00855-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marsischky GT, Filosi N, Kane MF, Kolodner R. Redundancy of Saccharomyces cerevisiae MSH3 and MSH6 in MSH2-dependent mismatch repair. Genes Dev. 1996;10:407–420. doi: 10.1101/gad.10.4.407. [DOI] [PubMed] [Google Scholar]
- 17.Wilson T, Guerrette S, Fishel R. Dissociation of mismatch recognition and ATPase activity by hMSH2-hMSH3. J Biol Chem. 1999;274:21659–21644. doi: 10.1074/jbc.274.31.21659. [DOI] [PubMed] [Google Scholar]
- 18.Lamers MH, Perrakis A, Enzlin JH, Winterwerp HH, de Wind N, Sixma TK. The crystal structure of DNA mismatch repair protein MutS binding to a G × T mismatch. Nature. 2000;407:711–717. doi: 10.1038/35037523. [DOI] [PubMed] [Google Scholar]
- 19.Obmolova G, Ban C, Hsieh P, Yang W. Crystal structures of mismatch repair protein MutS and its complex with a substrate DNA. Nature. 2000;407:703–710. doi: 10.1038/35037509. [DOI] [PubMed] [Google Scholar]
- 20.Heinen CD, Cyr JL, Cook C, Punja N, Sakato M, Forties RA, Lopez JM, Hingorani MM, Fishel R. Human MSH2 (hMSH2) protein controls ATP processing by hMSH2-hMSH6. J Biol Chem. 2011;286:40287–40295. doi: 10.1074/jbc.M111.297523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lamers MH, Winterwerp HH, Sixma TK. The alternating ATPase domains of MutS control DNA mismatch repair. EMBO J. 2003;22:746–756. doi: 10.1093/emboj/cdg064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mazur DJ, Mendillo ML, Kolodner RD. Inhibition of Msh6 ATPase activity by mispaired DNA induces a Msh2(ATP)-Msh6(ATP) state capable of hydrolysis-independent movement along DNA. Mol Cell. 2006;22:39–49. doi: 10.1016/j.molcel.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 23.Acharya S, Foster PL, Brooks P, Fishel R. The coordinated functions of the E. coli MutS and MutL proteins in mismatch repair. Mol Cell. 2003;12:233–246. doi: 10.1016/s1097-2765(03)00219-3. [DOI] [PubMed] [Google Scholar]
- 24.Gradia S, Acharya S, Fishel R. The human mismatch recognition complex hMSH2-hMSH6 functions as a novel molecular switch. Cell. 1997;91:995–1005. doi: 10.1016/s0092-8674(00)80490-0. [DOI] [PubMed] [Google Scholar]
- 25.Grilley M, Welsh KM, Su SS, Modrich P. Isolation and characterization of the Escherichia coli mutL gene product. J Biol Chem. 1989;264:1000–1004. [PubMed] [Google Scholar]
- 26.Ban C, Yang W. Crystal structure and ATPase activity of MutL: implications for DNA repair and mutagenesis. Cell. 1998;95:541–552. doi: 10.1016/s0092-8674(00)81621-9. [DOI] [PubMed] [Google Scholar]
- 27.Dutta R, Inouye M. GHKL, an emergent ATPase/kinase superfamily. Trends Biochem Sci. 2000;25:24–28. doi: 10.1016/s0968-0004(99)01503-0. [DOI] [PubMed] [Google Scholar]
- 28.Acharya S, Fishel R. The Mechanism of DNA Mismatch Repair from Bacteria to Human. Taylor & Francis Group; New York, NY: 2006. [Google Scholar]
- 29.Jiricny J. The multifaceted mismatch-repair system. Nat Rev Mol Cell Biol. 2006;7:335–346. doi: 10.1038/nrm1907. [DOI] [PubMed] [Google Scholar]
- 30.Kolodner RD, Marsischky GT. Eukaryotic DNA mismatch repair. Curr Opin Genet Dev. 1999;9:89–96. doi: 10.1016/s0959-437x(99)80013-6. [DOI] [PubMed] [Google Scholar]
- 31.Modrich P. Strand-specific mismatch repair in mammalian cells. J Biol Chem. 1997;272:24727–24730. doi: 10.1074/jbc.272.40.24727. [DOI] [PubMed] [Google Scholar]
- 32.Marinus MG. Methylation of prokaryotic DNA. In: Razin A, Cedar H, Riggs AD, editors. DNA Methylation, Biochemistry and Biological Significance. Springer-Verlag; New York: 1984. pp. 81–109. [Google Scholar]
- 33.Viswanathan M, Lovett ST. Single-strand DNA-specific exonucleases in Escherichia coli – roles in repair and mutation avoidance. Genetics. 1998;149:7–16. doi: 10.1093/genetics/149.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Constantin N, Dzantiev L, Kadyrov FA, Modrich P. Human mismatch repair: reconstitution of a nick-directed bidirectional reaction. J Biol Chem. 2005;280:39752–39761. doi: 10.1074/jbc.M509701200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grilley M, Griffith J, Modrich P. Bidirectional excision in methyl-directed mismatch repair. J Biol Chem. 1993;268:11830–11837. [PubMed] [Google Scholar]
- 36.Lahue RS, Au KG, Modrich P. DNA mismatch correction in a defined system. Science. 1989;245:160–164. doi: 10.1126/science.2665076. [DOI] [PubMed] [Google Scholar]
- 37.Ghodgaonkar MM, Lazzaro F, Olivera-Pimentel M, Artola-Boran M, Cejka P, Reijns MA, Jackson AP, Plevani P, Muzi-Falconi M, Jiricny J. Ribonucleotides misincorporated into DNA act as strand-discrimination signals in eukaryotic mismatch repair. Mol Cell. 2013;50:323–332. doi: 10.1016/j.molcel.2013.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lujan SA, Williams JS, Clausen AR, Clark AB, Kunkel TA. Ribonucleotides are signals for mismatch repair of leading-strand replication errors. Mol Cell. 2013;50:437–443. doi: 10.1016/j.molcel.2013.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schmutte C, Marinescu RC, Sadoff MM, Guerrette S, Overhauser J, Fishel R. Human exonuclease I interacts with the mismatch repair protein hMSH2. Cancer Res. 1998;58:4537–4542. [PubMed] [Google Scholar]
- 40.Tishkoff DX, Amin NS, Viars CS, Arden KC, Kolodner RD. Identification of a human gene encoding a homologue of Saccharomyces cerevisiae EXO1, an exonuclease implicated in mismatch repair and recombination. Cancer Res. 1998;58:5027–5031. [PubMed] [Google Scholar]
- 41.Tishkoff DX, Boerger AL, Bertrand P, Filosi N, Gaida GM, Kane MF, Kolodner RD. Identification and characterization of Saccharomyces cerevisiae EXO1, a gene encoding an exonuclease that interacts with MSH2. Proc Natl Acad Sci U S A. 1997;94:7487–7492. doi: 10.1073/pnas.94.14.7487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang Y, Yuan F, Presnell SR, Tian K, Gao Y, Tomkinson AE, Gu L, Li GM. Reconstitution of 5′-directed human mismatch repair in a purified system. Cell. 2005;122:693–705. doi: 10.1016/j.cell.2005.06.027. [DOI] [PubMed] [Google Scholar]
- 43.Dzantiev L, Constantin N, Genschel J, Iyer RR, Burgers PM, Modrich P. A defined human system that supports bidirectional mismatch-provoked excision. Mol Cell. 2004;15:31–41. doi: 10.1016/j.molcel.2004.06.016. [DOI] [PubMed] [Google Scholar]
- 44.Kadyrov FA, Dzantiev L, Constantin N, Modrich P. Endonucleolytic function of MutLalpha in human mismatch repair. Cell. 2006;126:297–308. doi: 10.1016/j.cell.2006.05.039. [DOI] [PubMed] [Google Scholar]
- 45.Kadyrov FA, Holmes SF, Arana ME, Lukianova OA, O'Donnell M, Kunkel TA, Modrich P. Saccharomyces cerevisiae MutLalpha is a mismatch repair endonuclease. J Biol Chem. 2007;282:37181–37190. doi: 10.1074/jbc.M707617200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pillon MC, Lorenowicz JJ, Uckelmann M, Klocko AD, Mitchell RR, Chung YS, Modrich P, Walker GC, Simmons LA, Friedhoff P, Guarne A. Structure of the endonuclease domain of MutL: unlicensed to cut. Mol Cell. 2010;39:145–151. doi: 10.1016/j.molcel.2010.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pillon MC, Miller JH, Guarne A. The endonuclease domain of MutL interacts with the beta sliding clamp. DNA Repair (Amst) 2011;10:87–93. doi: 10.1016/j.dnarep.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Amin NS, Nguyen MN, Oh S, Kolodner RD. exo1-Dependent mutator mutations: model system for studying functional interactions in mismatch repair. Mol Cell Biol. 2001;21:5142–5155. doi: 10.1128/MCB.21.15.5142-5155.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wei K, Clark AB, Wong E, Kane MF, Mazur DJ, Parris T, Kolas NK, Russell R, Hou H, Jr, Kneitz B, Yang G, Kunkel TA, Kolodner RD, Cohen PE, Edelmann W. Inactivation of Exonuclease 1 in mice results in DNA mismatch repair defects, increased cancer susceptibility, and male and female sterility. Genes Dev. 2003;17:603–614. doi: 10.1101/gad.1060603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kolodner RD, Mendillo ML, Putnam CD. Coupling distant sites in DNA during DNA mismatch repair. Proc Natl Acad Sci U S A. 2007;104:12953–12954. doi: 10.1073/pnas.0705698104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fishel R, Acharya S, Berardini M, Bocker T, Charbonneau N, Cranston A, Gradia S, Guerrette S, Heinen CD, Mazurek A, Snowden T, Schmutte C, Shim KS, Tombline G, Wilson T. Signaling mismatch repair: the mechanics of an adenosine-nucleotide molecular switch, Cold Spring Harbor Symp. Quant Biol. 2000;65:217–224. doi: 10.1101/sqb.2000.65.217. [DOI] [PubMed] [Google Scholar]
- 52.Gradia S, Subramanian D, Wilson T, Acharya S, Makhov A, Griffith J, Fishel R. hMSH2-hMSH6 forms a hydrolysis-independent sliding clamp on mismatched DNA. Mol Cell. 1999;3:255–261. doi: 10.1016/s1097-2765(00)80316-0. [DOI] [PubMed] [Google Scholar]
- 53.Allen DJ, Makhov A, Grilley M, Taylor J, Thresher R, Modrich P, Griffith JD. MutS mediates heteroduplex loop formation by a translocation mechanism. EMBO J. 1997;16:4467–4476. doi: 10.1093/emboj/16.14.4467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Blackwell LJ, Bjornson KP, Modrich P. DNA-dependent activation of the hMutS alpha ATPase. J Biol Chem. 1998;273:32049–32054. doi: 10.1074/jbc.273.48.32049. [DOI] [PubMed] [Google Scholar]
- 55.Fishel R. Mismatch repair, molecular switches, and signal transduction. Genes Dev. 1998;12:2096–2101. doi: 10.1101/gad.12.14.2096. [DOI] [PubMed] [Google Scholar]
- 56.Junop MS, Obmolova G, Rausch K, Hsieh P, Yang W. Composite active site of an ABC ATPase: MutS uses ATP to verify mismatch recognition and authorize DNA repair. Mol Cell. 2001;7:1–12. doi: 10.1016/s1097-2765(01)00149-6. [DOI] [PubMed] [Google Scholar]
- 57.Pluciennik A, Modrich P. Protein roadblocks and helix discontinuities are barriers to the initiation of mismatch repair. Proc Natl Acad Sci U S A. 2007;104:12709–12713. doi: 10.1073/pnas.0705129104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Warren JJ, Pohlhaus TJ, Changela A, Iyer RR, Modrich PL, Beese LS. Structure of the human MutSalpha DNA lesion recognition complex. Mol Cell. 2007;26:579–592. doi: 10.1016/j.molcel.2007.04.018. [DOI] [PubMed] [Google Scholar]
- 59.Heinen CD, Wilson T, Mazurek A, Berardini M, Butz C, Fishel R. HNPCC mutations in hMSH2 result in reduced hMSH2-hMSH6 molecular switch functions. Cancer Cell. 2002;1:469–478. doi: 10.1016/s1535-6108(02)00073-9. [DOI] [PubMed] [Google Scholar]
- 60.Mendillo ML, Putnam CD, Mo AO, Jamison JW, Li S, Woods VL, Jr, Kolodner RD. Probing DNA- and ATP-mediated conformational changes in the MutS family of mispair recognition proteins using deuterium exchange mass spectrometry. J Biol Chem. 2010;285:13170–13182. doi: 10.1074/jbc.M110.108894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shell SS, Putnam CD, Kolodner RD. The N terminus of Saccharomyces cerevisiae Msh6 is an unstructured tether to PCNA. Mol Cell. 2007;26:565–578. doi: 10.1016/j.molcel.2007.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mirny L, Slutsky M, Wunderlich Z, Tafvizi A, Leith J, Kosmrlj A. How a protein searches for its site on DNA: the mechanism of facilitated diffusion. J Phys A: Math Theor. 2009;42:434013. [Google Scholar]
- 63.Berg OG, von Hippel PH. Diffusion-controlled macromolecular interactions. Annu Rev Biophys Biophys Chem. 1985;14:131–160. doi: 10.1146/annurev.bb.14.060185.001023. [DOI] [PubMed] [Google Scholar]
- 64.Berg OG, Winter RB, von Hippel PH. Diffusion-driven mechanisms of protein translocation on nucleic acids. 1. Models and theory. Biochemistry. 1981;20:6929–6948. doi: 10.1021/bi00527a028. [DOI] [PubMed] [Google Scholar]
- 65.Slutsky M, Kardar M, Mirny LA. Diffusion in correlated random potentials, with applications to DNA. Phys Rev E Stat Nonlin Soft Matter Phys. 2004;69:061903. doi: 10.1103/PhysRevE.69.061903. [DOI] [PubMed] [Google Scholar]
- 66.Slutsky M, Mirny LA. Kinetics of protein–DNA interaction: facilitated target location in sequence-dependent potential. Biophys J. 2004;87:4021–4035. doi: 10.1529/biophysj.104.050765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Blainey PC, van Oijen AM, Banerjee A, Verdine GL, Xie XS. A base-excision DNA-repair protein finds intrahelical lesion bases by fast sliding in contact with DNA. Proc Natl Acad Sci U S A. 2006;103:5752–5757. doi: 10.1073/pnas.0509723103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yanagida T, Ishii Y. Single Molecule Dynamics in Life Science. Wiley-VCH Verlag GmbH & Co; Weinheim, FRG: 2009. [Google Scholar]
- 69.Jiang J, Bai L, Surtees JA, Gemici Z, Wang MD, Alani E. Detection of high-affinity and sliding clamp modes for MSH2-MSH6 by single-molecule unzipping force analysis. Mol Cell. 2005;20:771–781. doi: 10.1016/j.molcel.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 70.Mendillo ML, Mazur DJ, Kolodner RD. Analysis of the interaction between the Saccharomyces cerevisiae MSH2-MSH6 and MLH1-PMS1 complexes with DNA using a reversible DNA end-blocking system. J Biol Chem. 2005;280:22245–22257. doi: 10.1074/jbc.M407545200. [DOI] [PubMed] [Google Scholar]
- 71.Gorman J, Chowdhury A, Surtees JA, Shimada J, Reichman DR, Alani E, Greene EC. Dynamic basis for one-dimensional DNA scanning by the mismatch repair complex Msh2-Msh6. Mol Cell. 2007;28:359–370. doi: 10.1016/j.molcel.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gorman J, Plys AJ, Visnapuu ML, Alani E, Greene EC. Visualizing one-dimensional diffusion of eukaryotic DNA repair factors along a chromatin lattice. Nat Struct Mol Biol. 2010;17:932–938. doi: 10.1038/nsmb.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Joo C, Balci H, Ishitsuka Y, Buranachai C, Ha T. Advances in single-molecule fluorescence methods for molecular biology. Annu Rev Biochem. 2008;77:51–76. doi: 10.1146/annurev.biochem.77.070606.101543. [DOI] [PubMed] [Google Scholar]
- 74.Park J, Jeon Y, In D, Fishel R, Ban C, Lee JB. Single-molecule analysis reveals the kinetics and physiological relevance of MutL-ssDNA binding. PLoS ONE. 2010;5:e15496. doi: 10.1371/journal.pone.0015496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Drotschmann K, Hall MC, Shcherbakova PV, Wang H, Erie DA, Brownewell FR, Kool ET, Kunkel TA. DNA binding properties of the yeast Msh2-Msh6 and Mlh1-Pms1 heterodimers. Biol Chem. 2002;383:969–975. doi: 10.1515/BC.2002.103. [DOI] [PubMed] [Google Scholar]
- 76.Sass LE, Lanyi C, Weninger K, Erie DA. Single-molecule FRET TACKLE reveals highly dynamic mismatched DNA-MutS complexes. Biochemistry. 2010;49:3174–3190. doi: 10.1021/bi901871u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jeong C, Cho WK, Song KM, Cook C, Yoon TY, Ban C, Fishel R, Lee JB. MutS switches between two fundamentally distinct clamps during mismatch repair. Nat Struct Mol Biol. 2011;18:379–385. doi: 10.1038/nsmb.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cho WK, Jeong C, Kim D, Chang M, Song KM, Hanne J, Ban C, Fishel R, Lee JB. ATP alters the diffusion mechanics of MutS on mismatched DNA. Structure. 2012;20:1264–1274. doi: 10.1016/j.str.2012.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Qiu R, DeRocco VC, Harris C, Sharma A, Hingorani MM, Erie DA, Weninger KR. Large conformational changes in MutS during DNA scanning, mismatch recognition and repair signalling. EMBO J. 2012;31:2528–2540. doi: 10.1038/emboj.2012.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gorman J, Wang F, Redding S, Plys AJ, Fazio T, Wind S, Alani EE, Greene EC. Single-molecule imaging reveals target-search mechanisms during DNA mismatch repair. Proc Natl Acad Sci U S A. 2012;109:E3074–E3083. doi: 10.1073/pnas.1211364109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tessmer I, Yang Y, Zhai J, Du C, Hsieh P, Hingorani MM, Erie DA. Mechanism of MutS searching for DNA mismatches and signaling repair. J Biol Chem. 2008;283:36646–36654. doi: 10.1074/jbc.M805712200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schofield MJ, Nayak S, Scott TH, Du C, Hsieh P. Interaction of Escherichia coli MutS and MutL at a DNA mismatch. J Biol Chem. 2001;276:28291–28299. doi: 10.1074/jbc.M103148200. [DOI] [PubMed] [Google Scholar]
- 83.Yang Y, Sass LE, Du C, Hsieh P, Erie DA. Determination of protein-DNA binding constants and specificities from statistical analyses of single molecules: MutS-DNA interactions. Nucleic Acids Res. 2005;33:4322–4334. doi: 10.1093/nar/gki708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mazurek A, Johnson CN, Germann MW, Fishel R. Sequence context effect for hMSH2-hMSH6 mismatch-dependent activation. Proc Natl Acad Sci U S A. 2009;106:4177–4182. doi: 10.1073/pnas.0808572106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Forget AL, Kowalczykowski SC. Single-molecule imaging of DNA pairing by RecA reveals a three-dimensional homology search. Nature. 2012;482:423–427. doi: 10.1038/nature10782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Graneli A, Yeykal CC, Robertson RB, Greene EC. Long-distance lateral diffusion of human Rad51 on double-stranded DNA. Proc Natl Acad Sci U S A. 2006;103:1221–1226. doi: 10.1073/pnas.0508366103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tafvizi A, Huang F, Leith JS, Fersht AR, Mirny LA, van Oijen AM. Tumor suppressor p53 slides on DNA with low friction and high stability. Biophys J. 2008;95:L01–L03. doi: 10.1529/biophysj.108.134122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang YM, Austin RH, Cox EC. Single molecule measurements of repressor protein 1D diffusion on DNA. Phys Rev Lett. 2006;97:048302. doi: 10.1103/PhysRevLett.97.048302. [DOI] [PubMed] [Google Scholar]
- 89.Forties RA, North JA, Javaid S, Tabbaa OP, Fishel R, Poirier MG, Bund-schuh R. A quantitative model of nucleosome dynamics. Nucleic Acids Res. 2011;39:8306–8313. doi: 10.1093/nar/gkr422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Javaid S, Manohar M, Punja N, Mooney A, Ottesen JJ, Poirier MG, Fishel R. Nucleosome remodeling by hMSH2-hMSH6. Mol Cell. 2009;36:1086–1094. doi: 10.1016/j.molcel.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gruber HJ, Hahn CD, Kada G, Riener CK, Harms GS, Ahrer W, Dax TG, Knaus HG. Anomalous fluorescence enhancement of Cy3 and cy3.5 versus anomalous fluorescence loss of Cy5 and Cy7 upon covalent linking to IgG and noncovalent binding to avidin. Bioconjug Chem. 2000;11:696–704. doi: 10.1021/bc000015m. [DOI] [PubMed] [Google Scholar]
- 92.Iqbal A, Arslan S, Okumus B, Wilson TJ, Giraud G, Norman DG, Ha T, Lilley DM. Orientation dependence in fluorescent energy transfer between Cy3 and Cy5 terminally attached to double-stranded nucleic acids. Proc Natl Acad Sci U S A. 2008;105:11176–11181. doi: 10.1073/pnas.0801707105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Oiwa K, Jameson DM, Croney JC, Davis CT, Eccleston JF, Anson M. The 2′-O- and 3′-O-Cy3-EDA-ATP(ADP) complexes with myosin subfragment-1 are spectroscopically distinct. Biophys J. 2003;84:634–642. doi: 10.1016/S0006-3495(03)74883-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kunkel TA, Erie DA. DNA mismatch repair. Annu Rev Biochem. 2005;74:681–710. doi: 10.1146/annurev.biochem.74.082803.133243. [DOI] [PubMed] [Google Scholar]
- 95.Yang W. Poor base stacking at DNA lesions may initiate recognition by many repair proteins. DNA Repair (Amst) 2006;5:654–666. doi: 10.1016/j.dnarep.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 96.Antony E, Hingorani MM. Asymmetric ATP binding and hydrolysis activity of the Thermus aquaticus MutS dimer is key to modulation of its interactions with mismatched DNA. Biochemistry. 2004;43:13115–13128. doi: 10.1021/bi049010t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Blackwell LJ, Martik D, Bjornson KP, Bjornson ES, Modrich P. Nucleotide- promoted release of hMutS alpha from heteroduplex DNA is consistent with an ATP-dependent translocation Mechanism. J Biol Chem. 1998;273:32055–32062. doi: 10.1074/jbc.273.48.32055. [DOI] [PubMed] [Google Scholar]
- 98.Flores-Rozas H, Clark D, Kolodner RD. Proliferating cell nuclear antigen and Msh2p-Msh6p interact to form an active mispair recognition complex. Nat Genet. 2000;26:375–378. doi: 10.1038/81708. [DOI] [PubMed] [Google Scholar]
- 99.Gu L, Hong Y, McCulloch S, Watanabe H, Li GM. ATP-dependent interaction of human mismatch repair proteins and dual role of PCNA in mismatch repair. Nucleic Acids Res. 1998;26:1173–1178. doi: 10.1093/nar/26.5.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lau PJ, Kolodner RD. Transfer of the MSH2.MSH6 complex from proliferating cell nuclear antigen to mispaired bases in DNA. J Biol Chem. 2003;278:14–17. doi: 10.1074/jbc.C200627200. [DOI] [PubMed] [Google Scholar]
- 101.Pluciennik A, Dzantiev L, Iyer RR, Constantin N, Kadyrov FA, Modrich P. PCNA function in the activation and strand direction of MutLalpha endonuclease in mismatch repair. Proc Natl Acad Sci U S A. 2010;107:16066–16071. doi: 10.1073/pnas.1010662107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Umar A, Buermeyer AB, Simon JA, Thomas DC, Clark AB, Liskay RM, Kunkel TA. Requirement for PCNA in DNA mismatch repair at a step preceding DNA resynthesis. Cell. 1996;87:65–73. doi: 10.1016/s0092-8674(00)81323-9. [DOI] [PubMed] [Google Scholar]
- 103.Lee JB, Hite RK, Hamdan SM, Xie XS, Richardson CC, van Oijen AM. DNA primase acts as a molecular brake in DNA replication. Nature. 2006;439:621–624. doi: 10.1038/nature04317. [DOI] [PubMed] [Google Scholar]
- 104.Elez M, Radman M, Matic I. Stoichiometry of MutS and MutL at unrepaired mismatches in vivo suggests a mechanism of repair. Nucleic Acids Res. 2012;40:3929–3938. doi: 10.1093/nar/gkr1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hombauer H, Campbell CS, Smith CE, Desai A, Kolodner RD. Visualization of eukaryotic DNA mismatch repair reveals distinct recognition and repair intermediates. Cell. 2011;147:1040–1053. doi: 10.1016/j.cell.2011.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gebhardt JC, Suter DM, Roy R, Zhao ZW, Chapman AR, Basu S, Maniatis T, Xie XS. Single-molecule imaging of transcription factor binding to DNA in live mammalian cells. Nat Methods. 2013;10:421–426. doi: 10.1038/nmeth.2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lia G, Michel B, Allemand JF. Polymerase exchange during Okazaki fragment synthesis observed in living cells. Science. 2012;335:328–331. doi: 10.1126/science.1210400. [DOI] [PubMed] [Google Scholar]
- 108.Wolter S, Schuttpelz M, Tscherepanow M, Van De Linde S, Heilemann M, Sauer M. Real-time computation of subdiffraction-resolution fluorescence images. J Microsc. 2010;237:12–22. doi: 10.1111/j.1365-2818.2009.03287.x. [DOI] [PubMed] [Google Scholar]
- 109.Henriques R, Lelek M, Fornasiero EF, Valtorta F, Zimmer C, Mhlanga MM. QuickPALM: 3D real-time photoactivation nanoscopy image processing in ImageJ. Nat Methods. 2010;7:339–340. doi: 10.1038/nmeth0510-339. [DOI] [PubMed] [Google Scholar]
- 110.Hedde PN, Fuchs J, Oswald F, Wiedenmann J, Nienhaus GU. Online image analysis software for photoactivation localization microscopy. Nat Methods. 2009;6:689–690. doi: 10.1038/nmeth1009-689. [DOI] [PubMed] [Google Scholar]
- 111.Lee S, Oh J, Kim D, Kim S, Lee JB, Nam HG. Polarization-controlled photoswitching resolves dipole directions with subwavelength resolution. Phys Rev Lett. 2012;109:28101. doi: 10.1103/PhysRevLett.109.248101. [DOI] [PubMed] [Google Scholar]
- 112.Biswas I, Hsieh P. Identification and characterization of a thermostable MutS homolog from Thermus aquaticus. J Biol Chem. 1996;271:5040–5048. doi: 10.1074/jbc.271.9.5040. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


