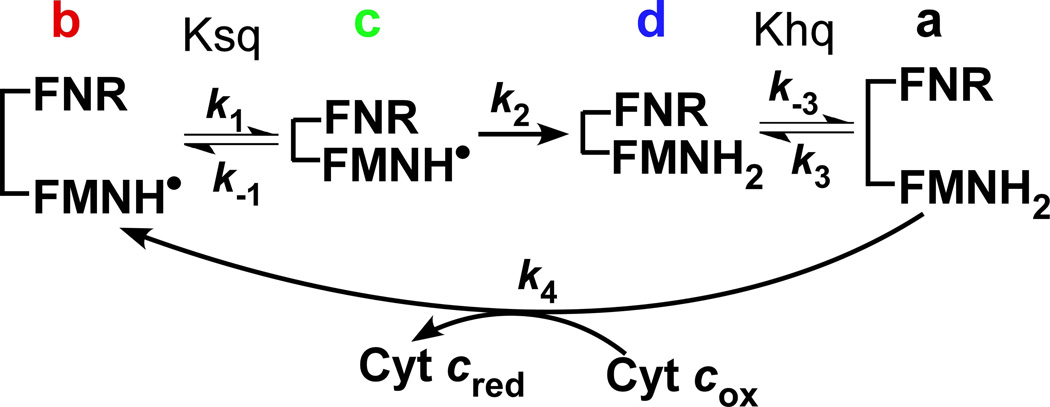
Figure 1. Kinetic model for electron flux through a dual-flavin enzyme.
The model uses four kinetic rates: association (k1 or k3) and dissociation (k−1 or k−3) of the FMN and FNR domains; the FMNH• reduction rate (k2), and the cytochrome c reduction rate (k4). The fully-reduced enzyme in the open conformation (species a) reduces cytochrome c and generates species b, which then undergoes successive conformational closing, interflavin electron transfer, and conformational opening steps to complete the cycle.