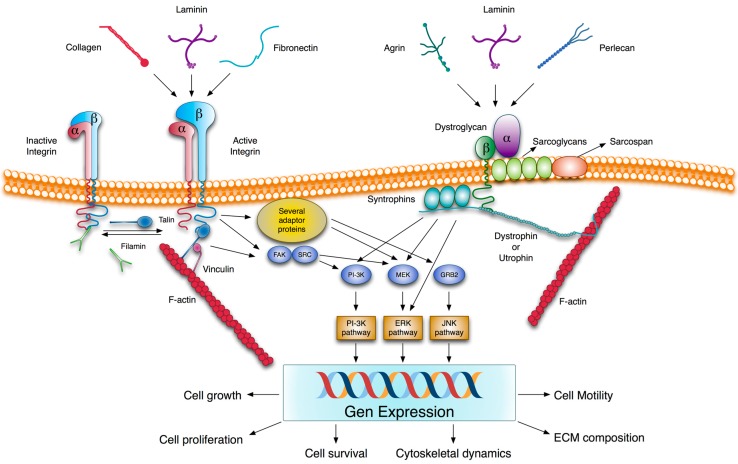
Figure 2.
Integrin- and dystroglycan-mediated ECM signaling. Several ECM components are able to interact with integrins and dystroglycans. Unlike dystroglycan, integrins are present on the cell surface as inactive and active forms. The conformational switch from low to high ligand affinity may be regulated by different ways. These include β-subunit cytoplasmic tail phosphorylation and competitive binding to this β-subunit tail between activators and inhibitors, such as cytoskeletal proteins talin and filamin, respectively. Ligand-integrin binding induces the recruitment of several adaptor proteins and the phosphorylation of some protein kinases such as focal adhesion kinase (FAK) which trigger different signaling pathways. In addition, integrins are linked to actin filaments through proteins, such as talin, filamin or actinin. Similarly, the interaction of α-dystroglycan with the LG domain containing ligands transduces extracellular information via β-dystroglycan to generate intracellular signals, which are mediated by signaling pathways, also activated by integrins. In fact, there is much evidence to suggest a cross-talk between both receptors. The signaling pathways induced by integrins and dystroglycans modulate gene expression patterns and impact cell behavior. The molecular organization of both receptors with their associated proteins provides a physical connection between the ECM and the cytoskeleton, and a way to transmit mechanical cues between them. ERK, extracellular signal-regulated kinase; FAK, focal adhesion kinase; GRB2, growth factor receptor-bound protein 2; JNK, c-Jun N-terminal kinase; MEK, MAPK kinase; PI-3K, phosphatidylinositol 3-kinase; Src, protein tyrosine kinase encoded by the c-src gene.