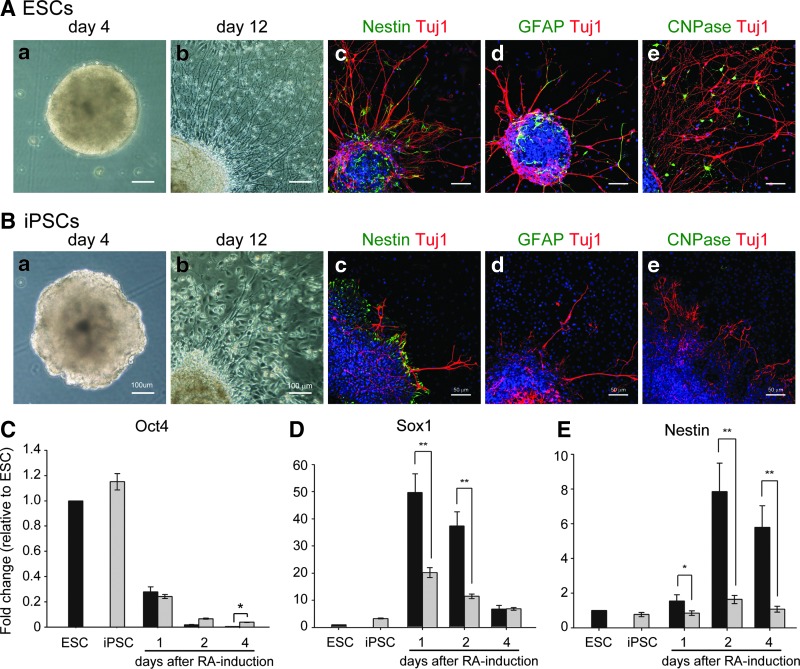
FIG. 1.
Neural differentiation of ESCs and iPSCs. Both ESCs and iPSCs formed EBs (Aa and Ba). ESC-derived EBs looked like spherical aggregates with a smooth surface (Aa); in contrast, iPSCs formed spherical aggregates with a rough surface (Ba). After plating EBs for 8 days, ESC- and iPSC-derived neurite outgrowth was observed by phase-contrast microscopy (Ab and Bb). Nestin-positive neural progenitors and Tuj1-positive neurons were detected in ESC- and iPSC-differentiated cells by immunocytochemical analysis using confocal microscopy (Nestin, Ac and Bc; Tuj1, Ac–e and Bc–e). GFAP-positive astrocytes and CNPase-positive oligodendrocytes were detected in ESC-differentiated cells (Ad and Ae), but were hardly found in iPSC-differentiated cells, as assessed by immunocytochemical analysis using confocal microscopy (Bd and Be). Nuclear staining with Hoechst 33342 was shown (Ac–e and Bc–e). Oct4 was rapidly downregulated in both ESCs and iPSCs after RA treatment (C). Sox1 expression in ESCs and iPSCs was first increased at day 1 after RA treatment, with expression levels that were markedly lower in iPSCs than they were in ESCs (D). Nestin expression in ESCs and iPSCs was rapidly increased at day 2 after RA treatment, with expression levels that were markedly lower in iPSCs compared with ESCs (E). Tuj1 expression in ESC- and iPSC-derived cells was upregulated after RA treatment, with expression levels that were significantly higher in ESC-derived cells than they were in iPSC-derived cells. Error bars represent the mean±SEM. (*) P<0.05, (**)P<0.01.