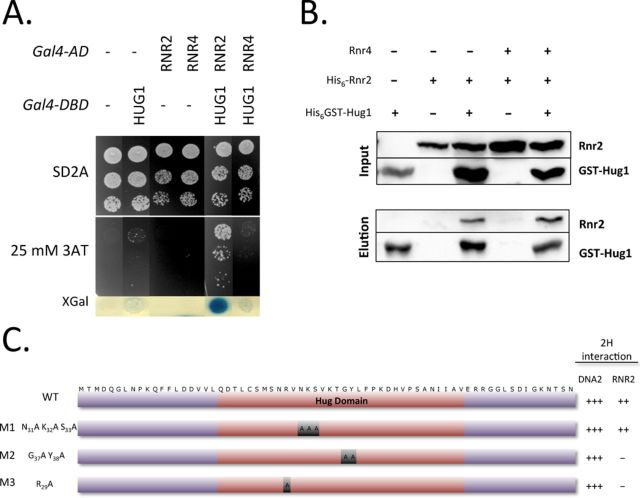
Figure 4.
Hug1 directly binds the Rnr2 ribonucleotide reductase subunit. (A) Hug1 was fused to Gal4 DNA-binding domain (Gal4-DBD) and Rnr2 or Rnr4 to Gal4 activating domain (Gal4-AD). Empty vectors (−) (pGBT9 and pACT2 respectively) were used as negative controls. Serial dilutions of diploids containing the various combinations of Gal4 fusions were plated in the absence (SD2A) or in the presence of indicated concentrations of 3-Amino-Triazol (3AT) to evaluate transcriptional activation of HIS3. Blue color formation in the presence of X-gal indicating transcriptional activation of the second reporter gene LacZ was then monitored. (B) Recombinant His-GST-Hug1 (GST-Hug1) was produced in E. coli cells. Soluble extracts were loaded onto Glutathion Agarose. Recombinant His6-Rnr2 expressed or His6-Rnr2−Rnr4 co-expressed was produced in E. coli cells. Soluble extracts of E. coli cells expressing His6-Rnr2 or co-expressing His6-Rnr2−Rnr4 were then added. After washing, bound proteins were eluted by adding GSH and analysed by western blotting. Extracts from E. coli containing the corresponding empty vectors (-) were used as controls. (C) Wild-type (WT) or mutant versions (M1, M2 or M3) of Hug1 were fused to Gal4-DBD. The transformants were mated with Y190 strain containing Gal4-AD-Rnr2 or Gal4-AD-Dna2. Growth of diploids cells was tested in the presence of various concentrations of 3AT to evaluate the HIS3 reporter activation. Blue color formation in the presence of X-Gal was measured to evaluate transcriptional activation of the LACZ reporter gene. Activation of reporter genes was very high (+++), high (++) or undectable (-). Hug domain is indicated in red.