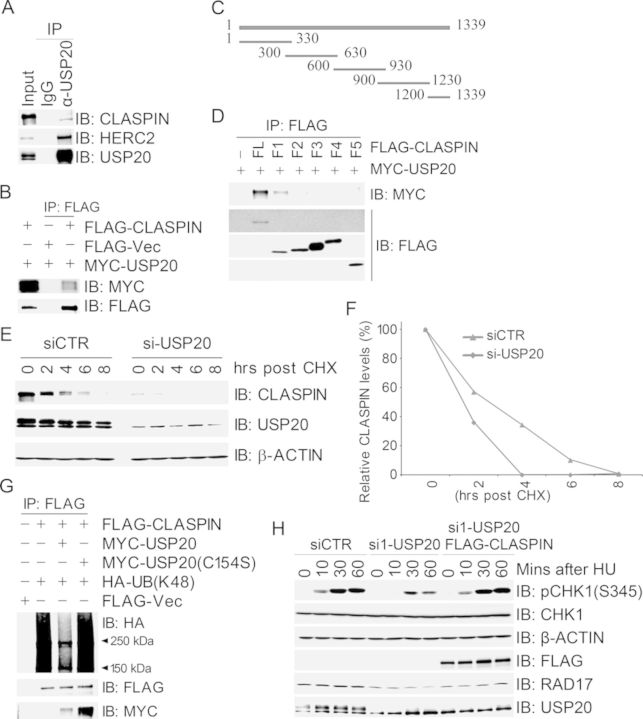
Figure 2.
USP20 promotes CLASPIN stability through deubiquitination. (A and B) USP20 interacted with CLASPIN. Total cell lysates were extracted from 293T cells (A) or 293T cells co-transfected with MYC-USP20 and FLAG-CLASPIN (B), and subjected to immunoprecipitation and immunoblotting with antibodies as indicated. (C) Schematic structure of CLASPIN fragments. (D) The N terminus of CLASPIN (1–330 AAs) mediated the interaction with USP20. Total cell lysates extracted from 293T cells co-transfected with MYC-USP20 and FLAG-CLASPIN or its fragments were subjected to immunoprecipitation with an anti-FLAG antibody followed by immunoblotting with an anti-MYC antibody. (E and F) Inhibition of USP20 expression decreased CLASPIN protein levels and shortened CLASPIN half-life. The siCTR- or si-USP20-transfected 293T cells were treated with CHX at different time points. Total cell lysates were harvested and used for immunoblotting with antibodies as indicated in (E), and quantification of the CLAPSIN/β-ACTIN ratio was plotted in (F). (G) USP20 deubiquitinated K48-linked polyubiquitination of CLASPIN in vivo. 293T cells were co-transfected with the expression constructs as indicated, total cell lysates were harvested 2 days later and subjected to immunoprecipitation followed by immunoblotting with antibodies as indicated. (H) USP20 depletion-induced delay of CHK1 activation in response to HU treatment was reversed by overexpression of CLASPIN. USP20-depleted 293T cells were transfected with FLAG-VEC or wild-type FLAG-CLASPIN and treated 2 days later with HU at different time points. Total cell lysates were extracted and used for immunoblotting with antibodies as indicated.