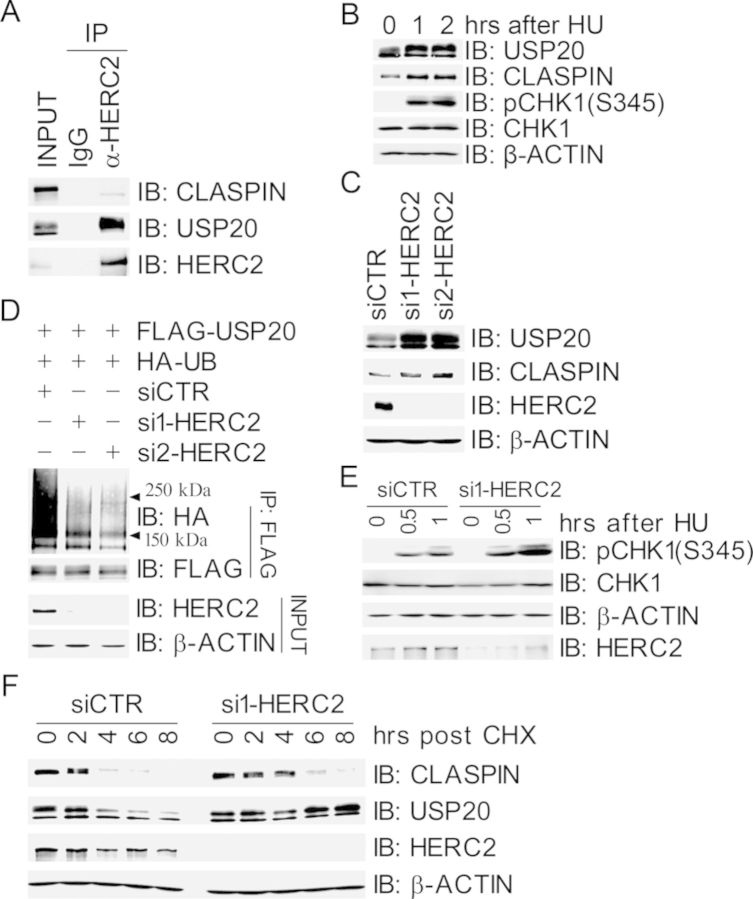
Figure 3.
HERC2 promotes USP20 ubiquitination and subsequent degradation. (A) USP20 interacted with HERC2. Total cell lysates were extracted from 293T cells and subjected to immunoprecipitation and immunoblotting with antibodies as indicated. (B) Both USP20 and CLASPIN protein levels increased in response to HU treatment. 293T cells were treated with HU at different time points as indicated, total cell lysates were extracted and subjected to immunoblotting with antibodies as indicated. (C) Inhibition of HERC2 expression increased protein levels of USP20 and CLASPIN. 293T cells were transfected with siCTR or HERC2-specific siRNA (si1-HERC2 and si2-HERC2). Total cell lysates were harvested 2 days later and subjected to immunoblotting with antibodies as indicated. (D) Inhibition of HERC2 expression decreased the ubiquitination levels of FLAG-USP20. HERC2-depleted 293T cells were co-transfected with expression constructs of FLAG-USP20 and HA-UB. Total cell lysates were harvested 2 days later and subjected to immunoprecipitation and immunoblotting with antibodies as indicated. (E) Depletion of HERC2 promotes HU-induced CHK1 activation. HERC2-depleted 293T cells were treated with HU at different time points as indicated. Total cell lysates were harvested and subjected to immunoblotting with antibodies as indicated. (F) Inhibition of HERC2 expression prolonged the half-life of USP20 and CLASPIN. Mocked- or HERC2-depleted 293T cells were treated with CHX at different time points as indicated, total cell lysates were harvested and subjected to immunoblotting with antibodies as indicated.