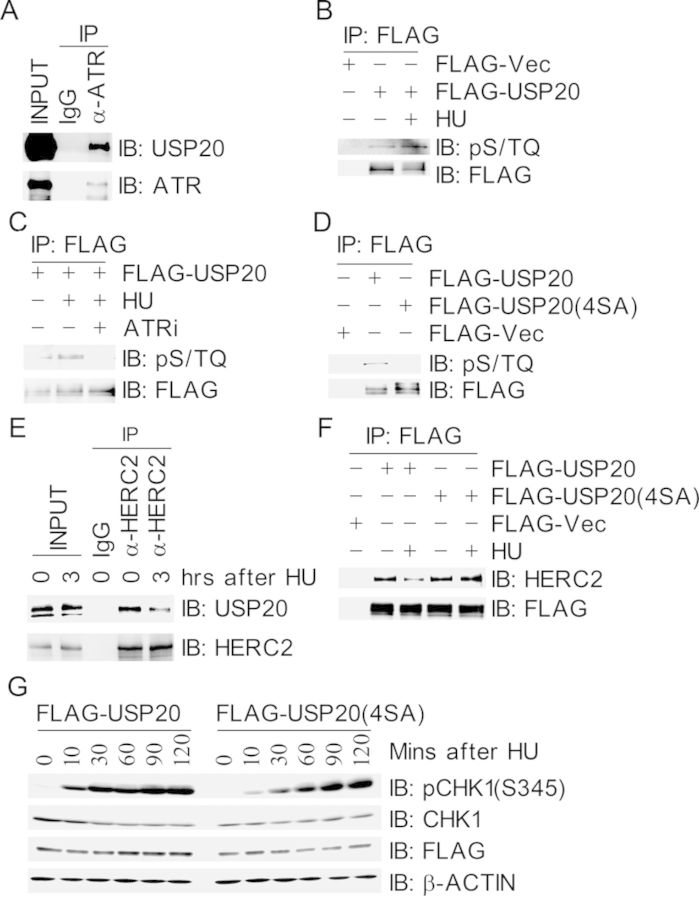
Figure 4.
ATR-mediated phosphorylation of USP20 promotes its dissociation from HERC2. (A) USP20 interacted with ATR. Total cell lysates were extracted from 293T cells and subjected to immunoprecipitation followed by immunoblotting with antibodies as indicated. (B and C) USP20 was phosphorylated by ATR in response to HU treatment. 293T cells expressing FLAG-USP20 were mocked-treated or treated with HU for 3 h (B) or pre-treated with the ATR inhibitor NU6027 (ATRi) for 1 h before HU treatment (C), total cells lysates were harvested and subjected to immunoprecipitation followed by immunoblotting with antibodies as indicated. (D) USP20 was phosphorylated on its S/TQ motifs. 293T cells were transfected with FLAG-VEC, FLAG-USP20 or FLAG-USP20(4SA), in which all the four S/TQ sites within the USP20 polypeptide were mutated to AQ. Total cells lysates were harvested 2 days later and subjected to immunoprecipitation followed by immunoblotting with antibodies as indicated. (E) The interaction between HERC2 and USP20 decreased under replication stress. Total cell lysates were extracted from 293T cells with mock treatment or HU treatment for 3 h and immunoprecipitated with an anti-HERC2 antibody. Immunoprecipitates were subjected to immunoblotting with antibodies as indicated. (F) Phosphorylation-deficient USP20(4SA) stably associated with HERC2 after HU treatment. 293T cells expressing FLAG-USP20 or FLAG-USP20(4SA) were treated with HU for 3 h, total cells lysates were harvested and subjected to immunoprecipitation followed by immunoblotting with antibodies as indicated. (G) Phosphorylation-deficient mutant USP20(4SA) delayed HU-induced CHK1 activation. 293T cells expressing FLAG-USP20 or FLAG-USP20(4SA) were treated with HU at different time points as indicated, and total cell lysates were extracted and used for immunoblotting with antibodies as indicated.