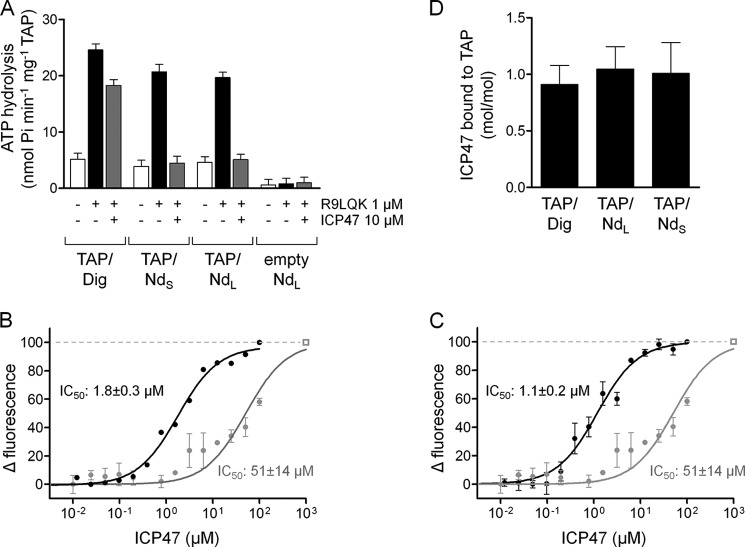
FIGURE 6.
Nanodiscs mimic the lipid environment required for high affinity viral inhibition of TAP. A, inhibition of ATP hydrolysis by the viral TAP inhibitor ICP47. TAP/Dig, TAP/NdS, or TAP/NdL was incubated with 5 mm MgCl2, 3 mm ATP, and either 1 μm R9LQK, 20 μm ICP47(2–34), or both. ATPase activity was measured for 30 min at 37 °C. B and C, competitive binding of peptide C4Atto655 and ICP47 was measured by MST. TAP/NdS (B) or TAP/NdL (C) (500 nm each) was incubated with C4Atto655 (25 nm) and increasing concentrations of ICP47(2–34). The IC50 values were calculated by the change in fluorescence with 1.8 ± 0.3 μm and c.i. 1.4–2.3 μm (TAP/NdS) and 1.1 ± 0.2 μm and c.i. 0.9–1.6 μm (TAP/NdL). The competitive peptide binding of ICP47 was compared with TAP/Dig, leading to an IC50 value of 51 ± 14 μm and c.i. of 34–76 μm (TAP/Dig, gray curves, identical data in B and C). The rectangle indicates the competition with a 300-fold excess of unlabeled peptide R9LQK. D, stoichiometry of ICP47 binding to TAP/Dig and TAP/Nd was determined by MC-FSEC at 0 °C. 1 or 12 μm of ICP47Atto565 were incubated with 500 nm TAP/Nd or TAP/Dig, respectively.