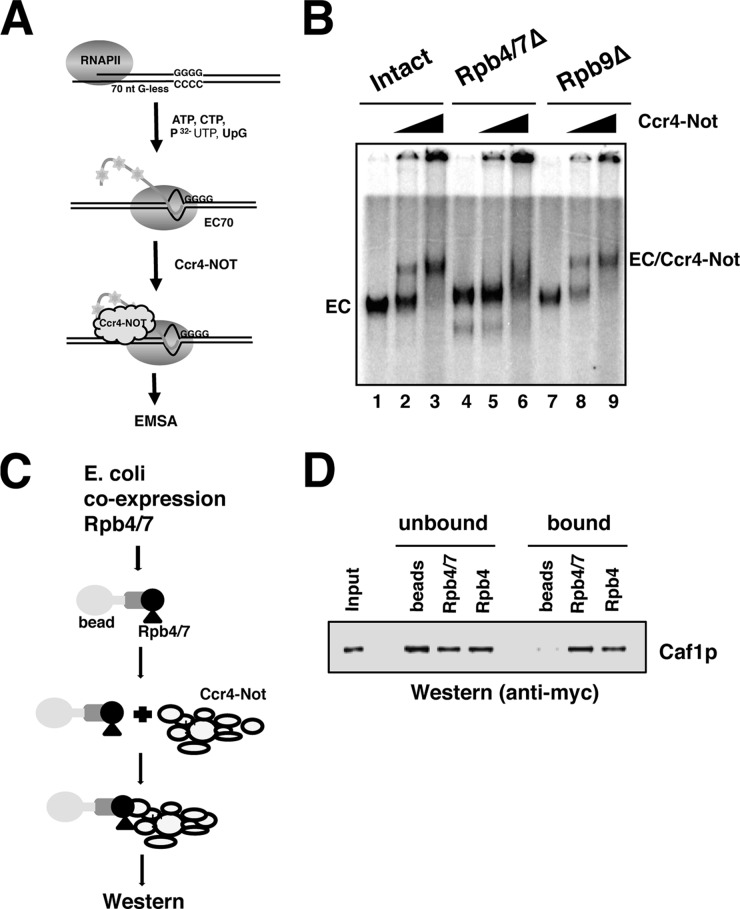
FIGURE 3.
Rpb4/7 is required for the stable association of Ccr4-Not with RNAPII elongation complexes. A, schematic of EC formation and binding assay. Approximately 250 fmol of intact or mutant RNAPII (Rpb4/7Δ and Rpb9Δ) was used to form ECs on 100 ng of template (EC70). ECs were incubated for 10 min with 0.5 and 1.5 μg of Ccr4-Not complex or carrier protein (RNAPII-only lanes). nt, nucleotides. B, products were separated on native polyacrylamide gels and visualized by the incorporation of radiolabeled UTP into the nascent transcript (see “Experimental Procedures”). C, schematic of the pulldown assay to measure the binding between recombinant Rpb4/7 and Ccr4-Not. Rpb4/7 were co-expressed using a bicistronic expression system, and Rpb4 was purified separately. Proteins (∼5 μg) were immobilized on Ni-NTA-agarose by a His6 tag incorporated into the Rpb4 subunit. Beads were incubated for 1 h with TAP-purified Ccr4-Not containing Myc-tagged Caf1. D, the fractions were analyzed by Western blotting using the 9E10 antibody. Naked beads were used as a control. 200 ng of purified Ccr4-Not is shown in the input lane. Bands were detected using a Cy-5-conjugated secondary antibody. Half of the total of each fraction was loaded onto the gel.