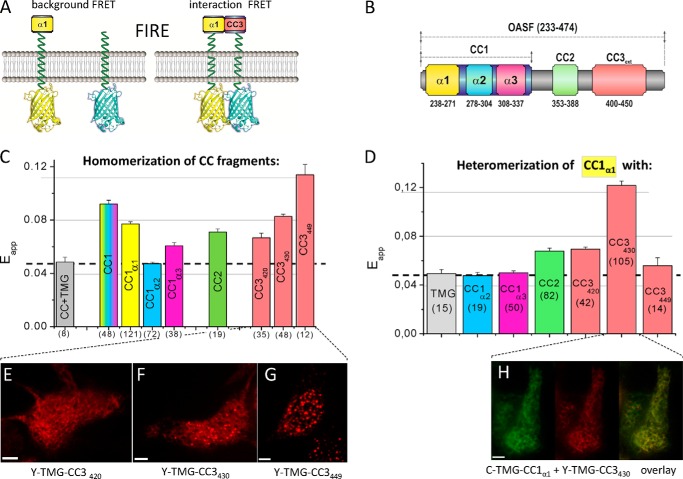
FIGURE 4.
Homomerization potential of coiled-coil domains as well as helical segments and their heteromerization with CC1α1. A, FIRE system: scheme of FIRE constructs illustrating background FRET (control, left) and FRET derived from a specific interaction (right). B, scheme of human STIM1 OASF depicting CC1, CC2 and CC3 domains. The same color coding for respective domains is used in C and D. C, homomerization potential of individual CC or helical fragments (CC1α1 aa 233–276; CC1α2 aa 273–309, CC1α3 aa 303–342) determined by FIRE. D, block diagram depicting heteromeric interactions, obtained by FIRE, of CC1α1 with: TMG (control), CC1α2, CC1α3, CC2, CC3420, CC3430, and CC3449. Dashed lines in C and D represent the magnitude of the background signal. Number of cells studied are given in brackets. E–G, representative fluorescence images of Y-TMG-CC3 constructs of various lengths: (E) Y-TMG-CC3420 (no cluster formation), (F) Y-TMG-CC3430 (partial cluster formation), and (G) Y-TMG-CC3449 (strong cluster formation). H, representative fluorescence images of co-expressed Y-TMG-CC1α1 + Y-TMG-CC3430.revealing ER distribution without cluster formation. Calibration bar is 5 μm throughout.