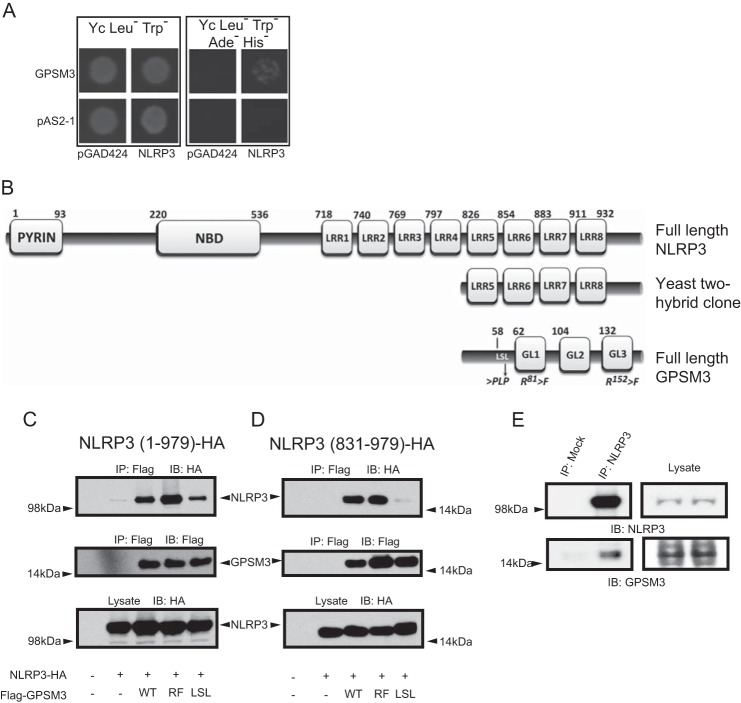
FIGURE 1.
Identification of NLRP3 as a GPSM3-interacting protein. A, Saccharomyces cerevisiae were co-transformed with indicated bait plasmids (either expressing the Gal4p DNA binding domain alone [pAS2-1] or as a fusion with full-length GPSM3 [pAS2-1/GPSM3]) and prey plasmids (either expressing the Gal4p activation domain alone [pGAD424] or as a fusion with the C terminus of NLRP3 [pACT2/NLRP3]). Transformed yeast were plated onto synthetic defined agar (Yc) lacking leucine (Leu−, to select for the prey plasmid) and tryptophan (Trp−, to select for the bait plasmid); growth on Yc Leu−Trp− medium demonstrates incorporation of both bait and prey plasmids (left panel). Growth on Yc Leu−Trp− medium also deficient in adenine (Ade−) and histidine (His−) indicates a positive protein-protein interaction (right panel). B, domain architecture of NLRP3 comprising the pyrin domain, nucleotide-binding domain (NBD), and eight leucine-rich repeats (LRRs) in comparison to the truncated domain structure for the NLRP3 fragment identified in the yeast two-hybrid screen. Additionally represented is the domain structure of GPSM3 comprising the three GoLoco motifs (GL1-GL3) and locations of point mutations used in this study. Amino acid numbering for each domain is based on data from UniProt identifier Q96P20 (NLRP3) and Q9Y4H4 (GPSM3). C and D, HEK293 cells were transiently co-transfected with plasmids expressing HA-tagged full-length NLRP3 (aa 1–979; panel C) or an NLRP3 C-terminal fragment (aa 831–979; panel D), along with Flag-tagged GPSM3, either of wild type sequence (WT), R81F + R152F double mutant (RF; abrogates GPSM3/Gαi·GDP interaction) or the LSL mutant in which the GPSM3 amino acids 58LSL were mutated to PLP to eliminate the GPSM3/Gβ interaction as previously described. Immunoprecipitation (IP) of GPSM3 was performed using agarose-conjugated anti-Flag M2 antibody and co-immunoprecipitating proteins were detected by immunoblotting (IB) with anti-HA epitope tag antibody. E, whole cell lysates from THP-1 cells were immunoprecipitated with anti-NLRP3 goat polyclonal antibody or with protein-A/-G agarose beads alone. GPSM3 was subsequently detected using an anti-GPSM3 mouse monoclonal antibody (clone 35.5.1) and NLRP3 was detected with anti-NLRP3 mouse monoclonal antibody. Results shown are representative of two independent experiments.