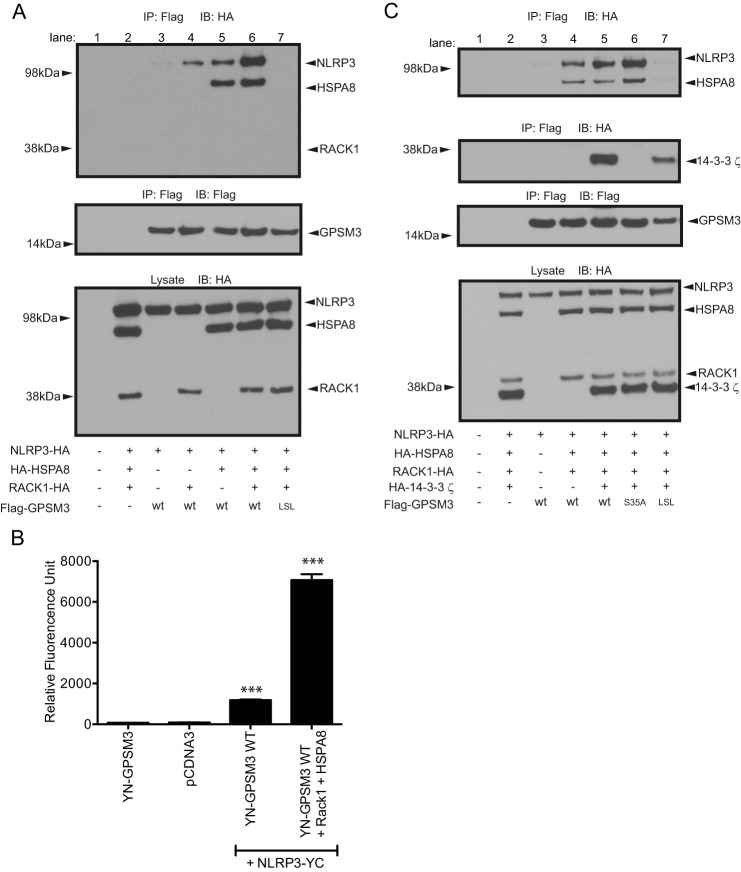
FIGURE 7.
The heat shock protein HSPA8 is involved in the interaction between NLRP3 and GPSM3. A, to assess potential roles of HSPA8 and RACK1 in the interaction between NLRP3 and GPSM3, HEK293 cells were transiently co-transfected with plasmids encoding Flag-tagged GPSM3, HA-tagged NLRP3, HA-tagged HSPA8, and/or C-terminal HA-tagged RACK1. Immunoprecipitation (IP) of GPSM3 performed using agarose-conjugate anti-Flag M2 antibody revealed the presence of HSPA8 in the complex, but the absence of RACK1, when immunoblotted with anti-HA. B, HEK293 cells were transfected with plasmids expressing YN-GPSM3 and NLRP3-YC in the presence or absence of RACK1 and HSPA8 and the fluorescence of reconstituted YFP fluorophore was measured at 24 h post-transfection. Cells were harvested and fluorescence quantified on a plate reader. Total DNA used for transfection was normalized using empty pcDNA3.1 vector DNA. Data are expressed as relative fluorescence units (RFU) using the means ± S.E. of at least three different experiments; *, p < 0.05; **, p < 0.01; and ***, p < 0.001 by one-way ANOVA. C, to assess the role of the known GPSM3 interactor 14-3-3, HEK293 cells were similarly co-transfected with Flag-tagged wild type GPSM3 or GPSM3 R35A mutant (a loss-of-function mutant abolishing interaction with 14-3-3; Ref. 20) and HA-tagged HSPA8 and HA-tagged 14-3-3.