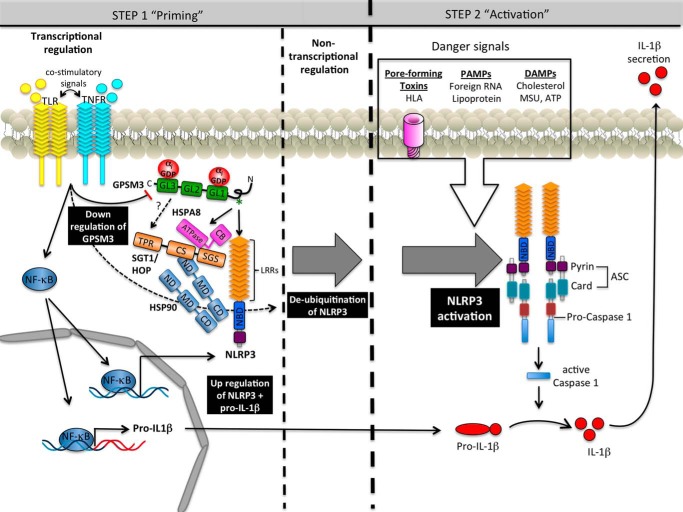
FIGURE 8.
Model of NLRP3 regulation by GPSM3. Formation of a network between GPSM3, chaperones, and NLRP3 maintains the steady-state level of NLRP3 by stabilizing it within this complex. Activation of the inflammasome requires two independent signals. Toll-like receptors (TLR) and tumor-necrosis factor receptors (TNFR) are known to trigger priming signals leading to up-regulation of NLRP3 and pro-IL1β through a NFκB-dependent pathway, as well as down-regulation of GPSM3 mRNA and protein levels. Non-transcriptional priming of NLRP3 allows inflammasome activation upon detection of the activation signal. The NLPR3 activation signal is triggered by endogenous DAMPs or exogenous PAMPs danger signals. Activation of NLRP3 can also be mediated by bacterial pore-forming toxins such as the HLA of S. aureus. Activation of NLRP3 leads to formation of a multiprotein complex containing oligomerized NLRP3, the adaptor ASC, and caspase-1. Once activated, caspase-1 cleaves pro-IL1β (and pro-IL-18), releasing the biological active form of IL-1β (and IL-18) and thereby triggering inflammation and antimicrobial activity.