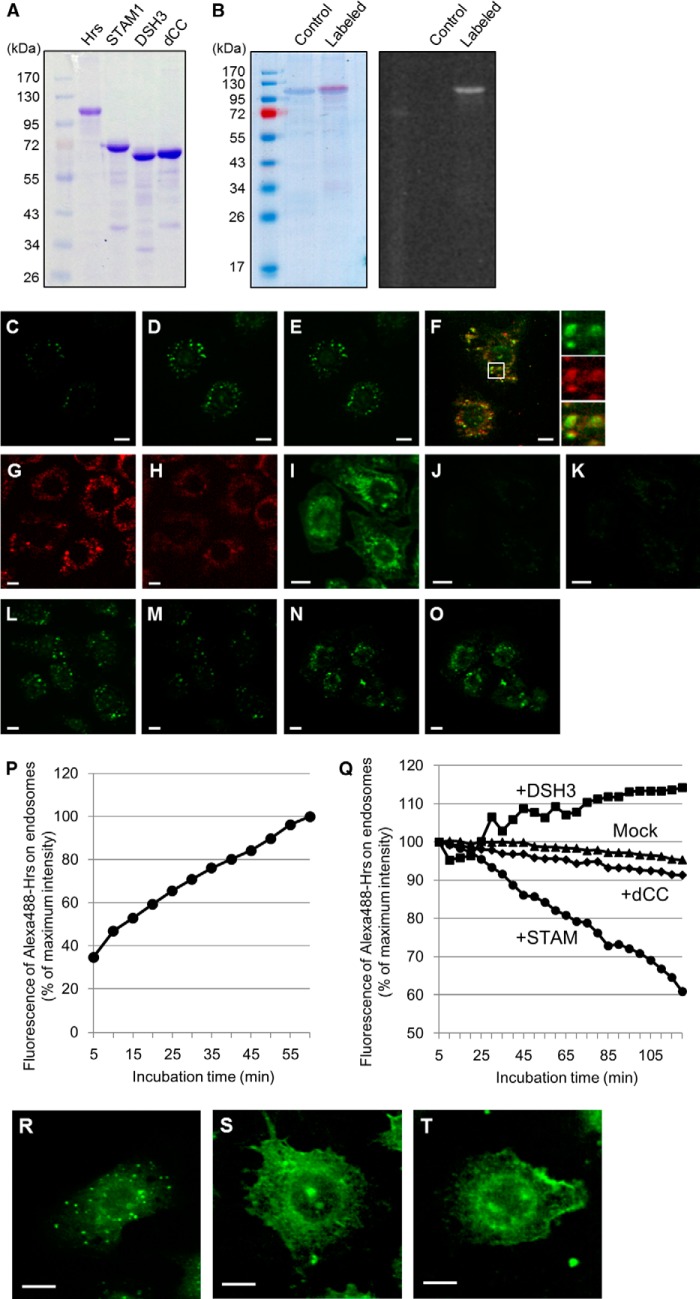
FIGURE 4.
STAM-independent endosomal targeting and STAM-mediated endosomal dissociation of Hrs. A, purification of the recombinant histidine-tagged Hrs, STAM1, and STAM1 mutants. The purified proteins were analyzed by SDS-PAGE with Coomassie Brilliant Blue staining. B, fluorescent labeling of the recombinant Hrs. The purified Hrs was labeled with the Alexa Fluor 488 dye. Coomassie Brilliant Blue-stained gel (left panel) and a UV-transilluminated (right panel) SDS-polyacrylamide gel after electrophoresis are shown. C and D, digitonin-permeabilized HRS knock-out mouse embryonic fibroblast HRSd cells were incubated in transport buffer (TB) containing 20 mm HEPES, pH 7.2, with Alexa Fluor 488-labeled Hrs (Alexa488-Hrs) at 37 °C. C and D represent the fluorescence images after 5- and 60-min incubation periods, respectively. The mean fluorescence intensity of the Alexa488-Hrs on endosomal punctate structures in semi-intact cells was quantified from the fluorescence images every 5 min for 1 h after Hrs loading (P). E, semi-intact cells in D were further incubated in TB buffer in the absence of Alexa488-Hrs and images captured every 5 min for the subsequent 2 h. The relative values of the mean intensity of Alexa488-Hrs fluorescence were plotted as the Mock dataset in Q. F, Alexa488-Hrs is targeted to the early endosome marker mRuby2-SARA-FYVE (mRuby2-FYVE)-positive endosomes in semi-intact HRSd cells. HRSd cells expressing mRuby2-FYVE (red) were permeabilized with digitonin and then treated with the TB-containing Alexa488-Hrs (green). The fluorescence image was captured after a 60-min incubation. G and H, neutralization of the intracellular pH elicits dissociation of the FYVE domain-containing proteins from the endosomal surface. Permeabilized HRSd cells expressing mRuby2-FYVE were treated with TB containing 100 mm HEPES, pH 7.2. G and H represent the fluorescence images before and after a 60-min incubation, respectively. I, analysis using the acidic organelles probe LysoSensor dye demonstrates the acidic environment of endosomes in semi-intact HRSd cells. J, intracellular environment of the cells in I is neutralized by treatment with TB containing 100 mm HEPES, pH 7.2. K, endosome-neutralized semi-intact cells in J were incubated in a TB containing 20 mm HEPES, pH 7.2, with Alexa488-Hrs at 37 °C for 1 h. L–O, effect of STAM1- and DSH3 mutant-loading on the endosomal localization of Hrs. Alexa488-Hrs loaded semi-intact cells, as shown in D, were further incubated in a TB containing 20 mm HEPES, pH 7.2, with recombinant STAM1 (L and M) and DSH3 mutants (N and O). The fluorescence images after a 5-min (L and N) and a 120-min incubation (M and O) are presented. The relative values of the mean intensity of Alexa488-Hrs fluorescence in STAM1-, DSH3-, and dCC-loaded semi-intact cells were plotted as the +STAM, +DSH3, and +dCC datasets, respectively, in Q. R–T, requirement for the complex formation of ESCRT-0 proteins on their endosomal targeting. Alexa488-Hrs was mixed with nonlabeled STAM1 or DSH3 mutants, and then microinjected into HRSd cells. After a 1-h incubation, the fluorescence images of the cells microinjected with Alexa488-Hrs alone as a control (R), the Alexa488-Hrs·STAM1 complex (S), or the Alexa488-Hrs/DSH3 mutant (T) were acquired. Scale bars, 10 μm.