FIGURE 1.
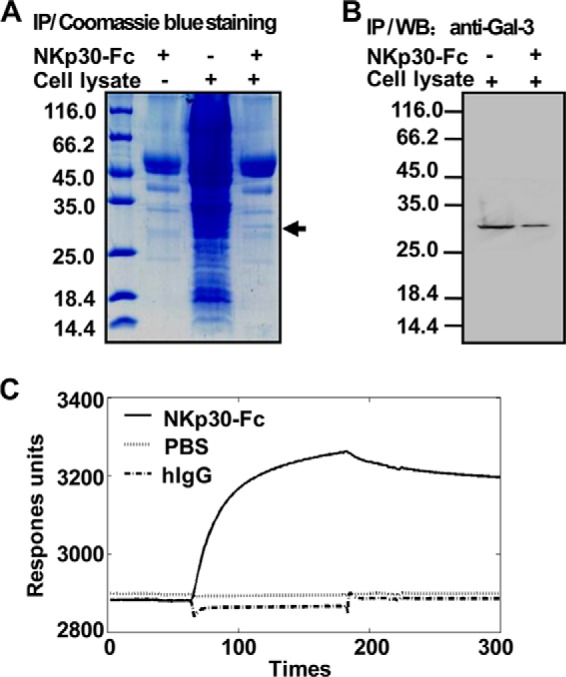
Interaction of Gal-3 with the extracellular domain of NKp30. A, binding of tumor-associated proteins to soluble NKp30-Fc. The NKp30-Fc fusion protein coupled to protein A/G-Sepharose beads were incubated with HeLa cell lysate, and the proteins that bound to NKp30-Fc were resolved by SDS-PAGE and stained with Coomassie Blue. An ∼30-kDa cellular protein that interacts with NKp30-Fc was present in the cell lysate. NKp30-Fc alone served as a negative control. IP, immunoprecipitation. B, binding of tumor-derived Gal-3 to soluble NKp30-Fc. Gal-3 protein or the cell lysate was examined by immunoblot analysis with an anti-Gal-3 mAb in immune complex. WB, Western blot. C, kinetics of the interactions between rhGal-3 and NKp30-Fc. rhGal-3 was covalently immobilized to the carboxyl groups in the dextran layer of a BIAcore sensor chip. Solutions containing NKp30-Fc or human IgG (hIgG) were injected over the surface of immobilized rhGal-3, and PBS buffer was used as a control. Surface plasmon resonance analysis was performed on a BIAcore 3000 apparatus at 25 °C. Under these conditions, binding of immobilized rhGal-3 to soluble NKp30-Fc was observed but binding to human IgG was not. The data are representative of two independent experiments.
