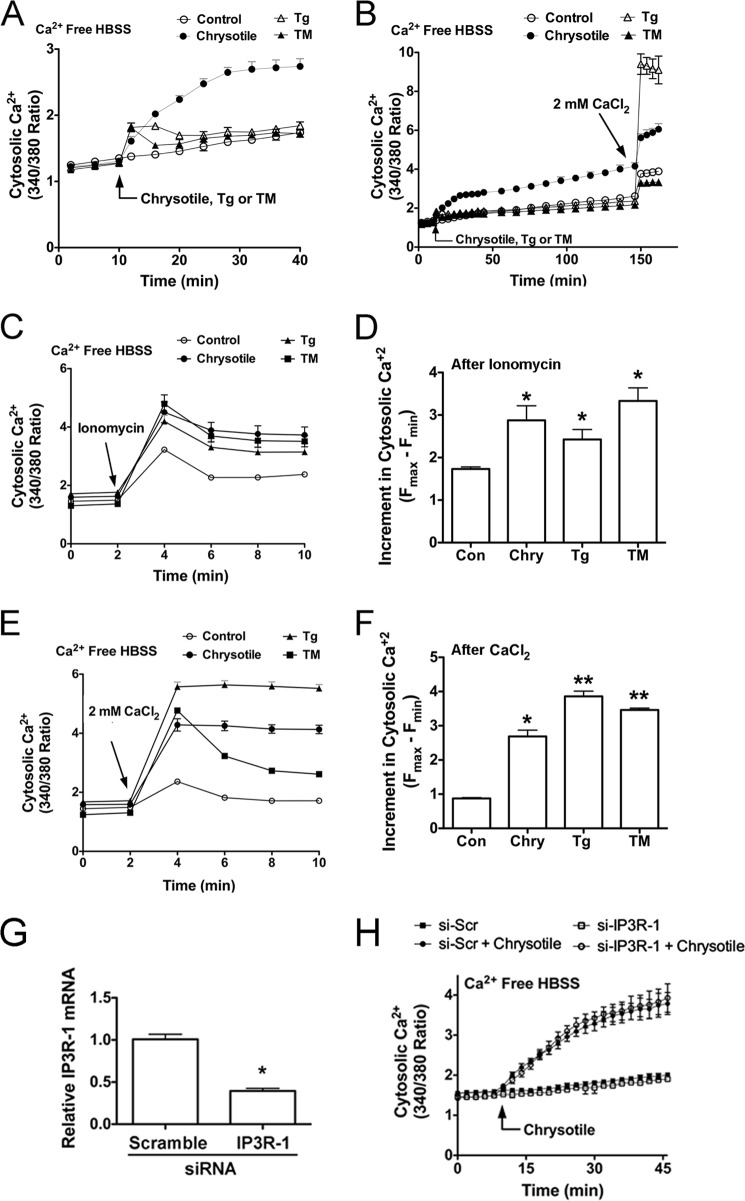
FIGURE 5.
Chrysotile increases ionomycin-releasable calcium stores and alters activation of store-operated Ca2+ channels in macrophages. A, cells were loaded with Fura2-AM (1 μm, 30 min, 37 °C), suspended in Ca2+-free HBSS, and exposed to chrysotile (10 μg/well), thapsigargin (Tg) (100 nm), or tunicamycin (TM) (5 μg/ml) followed by (B) 2 mm CaCl2. Values are mean ± S.E. (n = 6/group). C, cells were exposed to chrysotile (10 μg/well), thapsigargin (100 nm), or tunicamycin (5 μg/ml) for 24 h and then assayed for Ca2+ release by ionomycin (5 μm). D, increments in cytosolic Ca2+ were determined by the maximum change in F340/380 observed in the first 2 min after ionomycin. *, p < 0.05 versus control (n = 6/group). E, cells were exposed to chrysotile, thapsigargin, or tunicamycin for 24 h as described above, and then assayed for changes in cytosolic Ca2+ after addition of 2 mm CaCl2. F, increments in cytosolic Ca2+ after CaCl2 addition were determined as described in D. * or **, p < 0.05 versus control (n = 6/group). E, macrophages were transfected with either scrambled or IP3R-1 siRNA for 48 h and assayed for IP3R-1 mRNA by quantitative RT-PCR (*, p < 0.05 versus scramble) (G) and cytosolic Ca2+ levels after chrysotile exposure (H) (n = 6/group).