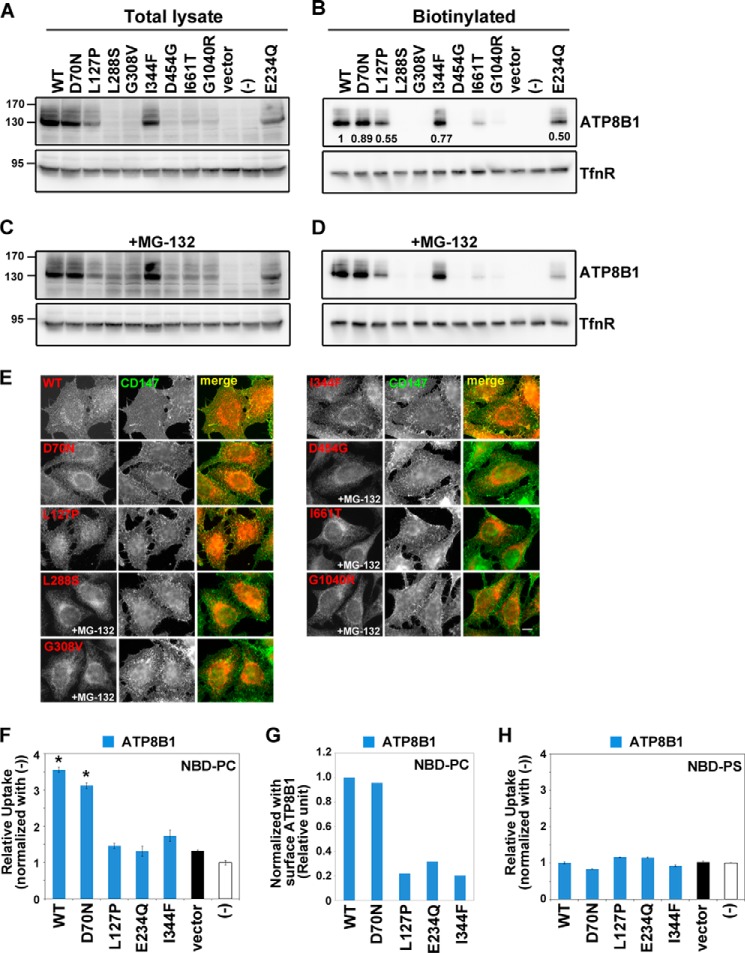
FIGURE 8.
Localization and flippase activity of ATP8B1 mutants associated with cholestasis. HeLa cell lines stably expressing the HA-tagged ATP8B1 mutants indicated were established by infection of recombinant retroviral vectors. A and C, the total expression level of each ATP8B1 protein was analyzed by immunoblotting with anti-HA and anti-TfnR antibodies. 4% of the input of the biotinylation reaction was loaded in each lane. B and D, cell surface expression level of each ATP8B1 protein was analyzed after surface biotinylation. The numbers in B show the relative expression level of proteins, which were normalized with the level of the internal control, TfnR. C and D, the cells were treated with 10 μm MG-132 for 12 h. E, cells expressing the indicated ATP8B1 protein were processed for immunostaining. Before fixation, cells were incubated with Alexa Fluor 488-conjugated anti-CD147 antibody for 5 min at room temperature to label the plasma membrane. The fixed cells were then incubated with anti-HA antibody followed by Cy3-conjugated anti-rat secondary antibody. Cells treated with MG-132 are indicated. Scale bar, 10 μm. Each cell line was incubated with NBD-PC (F) or NBD-PS (H) and then processed for the flippase assay as described in the legend for Fig. 4. -Fold increase of NBD-lipid uptake compared with parental HeLa cells (−) is shown. The graph is representative of two independent experiments, and results display averages from triplicates ±S.D. (*, p < 0.001). Error bars represent S.D. G, the PC flippase activities were normalized with the level of biotinylated P4-ATPases shown in B.