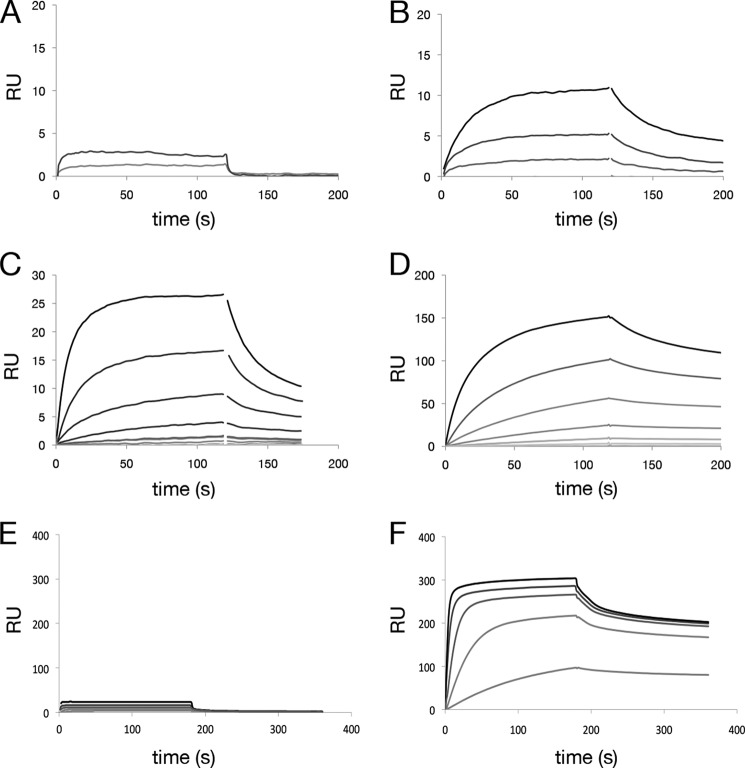
FIGURE 4.
Improvement in binding affinity for antibodies containing insertions. Improvement of antigen binding affinity for anti-hβNGF and anti-hGFRα1 antibodies containing CDRH1 and CDRL1 insertions, respectively. SPR was carried out by antibody capture at low density on an anti-human IgG surface followed by flowing antigen over the surface. SPR sensorgrams are shown for an anti-hβNGF antibody containing two point mutations, S31N and L45F in CDRH1 and FW2 (A); the same antibody with additionally incorporated insertions derived from in vitro SHM with affinity improvements of >20-fold (corresponding to Fig. 3A HC4-HC6) is shown (B–D). SPR sensorgrams for an anti-hGFRα1 antibody containing no mutations are shown in E and for the same antibody with a 5-amino acid insertion in CDRL1 (LC3 in Fig. 3B paired with parental HC), which confers an >40-fold improvement in affinity, in F. RU, response unit.