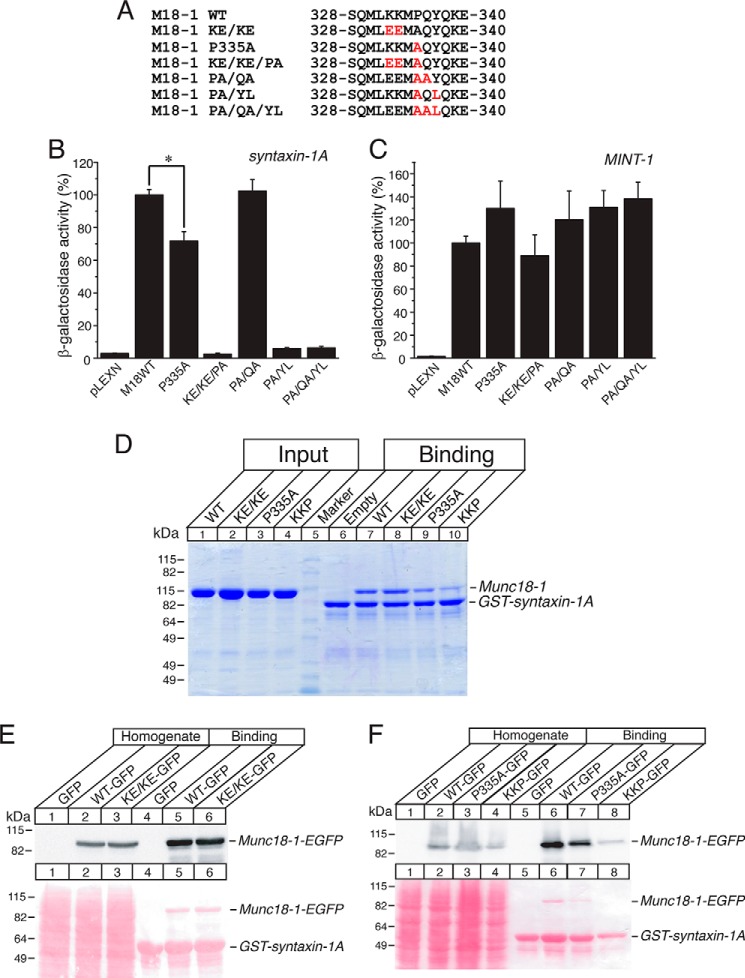
FIGURE 5.
The P335A mutant destabilizes the binding with monomeric closed syntaxin-1A. A, sequence alignment of the K332E/K33E/P335A (KE/KE/PA), P335A, P335A/Q336A (PA/QA), P335A/Y337L (PA/YL), and P335A/Q336A/Y337L (PA/QA/YL) mutants. M18-1, Munc18-1; KE/KE, K332E/K33E. B and C, the binding between Munc18-1 domain-3a mutants and syntaxin-1 (B) or Mint-1 (C) was analyzed by yeast two-hybrid assays. In this assay, β-galactosidase activities of the transformed yeast clones were quantified and normalized so that the activity of the yeast clones transformed with the wild-type Munc18-1 was set to 100%. Error bars indicate S.E. (n = 12–14 for syntaxin-1 interaction; n = 10–12 for Mint-1 interaction). *, p < 0.05. D–F, GST pulldown experiments testing the interactions between Munc18-1 domain-3a mutants (recombinant His6-Munc18-1 (D) or HEK-293FT expressed Munc18-1 (E and F)) with GST-fused cytosolic syntaxin-1A (1–264). D, the GST-syntaxin-1A pulled down Munc18-1 variants were shown as Coomassie Brilliant Blue staining. E and F, Ponceau S staining and immunoblotting with anti-Munc18-1 antibody. KKP, K332E/K333E/P335A.