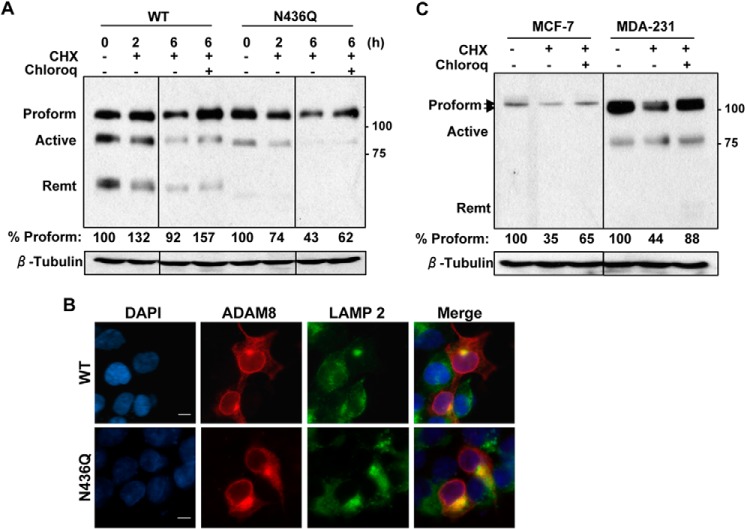
FIGURE 8.
N436Q is less stable than WT ADAM8 and degraded in the lysosomal compartment. A, HEK-293 cells were transiently transfected with WT ADAM8 or N436Q as in Fig. 4C. After 24 h, cells were treated with carrier DMSO (−), 25 μg/ml cycloheximide (CHX) in the absence (−) or presence (+) of 50 μm chloroquine (Chloroq) for 6 h. WCEs were analyzed by Western blotting for ADAM8 expression (using a Myc antibody) and for β-tubulin. All lanes were from the same gel, but cut to re-align as indicated by the vertical line. The values below are the amount of normalized ADAM8 Proform relative to the control sample (untreated sample), which was set to 100%, determined as described under “Experimental Procedures.” Results are from one of three representative blots with similar data. B, HEK-293 cells were transiently transfected with vectors expressing WT ADAM8 or its N-glycosylation mutant N436Q. Cells were fixed, permeabilized, and subjected to immunofluorescent staining using ADAM8 LSBio antibody (red); LAMP 2 (Lysosomal marker) (green), and DAPI (blue). Images were taken as described in Fig. 6A. Scale bar, 10 μm. C, MCF-7 and MDA-MB-231 cells were treated for 6 h with DMSO (−), cycloheximide and/or chloroquine, as above. WCEs were run on the same gel and analyzed for expression of endogenous ADAM8 (LSBio antibody) and β-tubulin by Western blotting. The values below are the average amounts of normalized ADAM8 Proform relative to the control sample, which was set to 100%, of three independent replicates. The vertical line indicates that the blots for MCF-7, and MDA-MB-231 cell lysates were exposed for different lengths of time.