FIGURE 9.
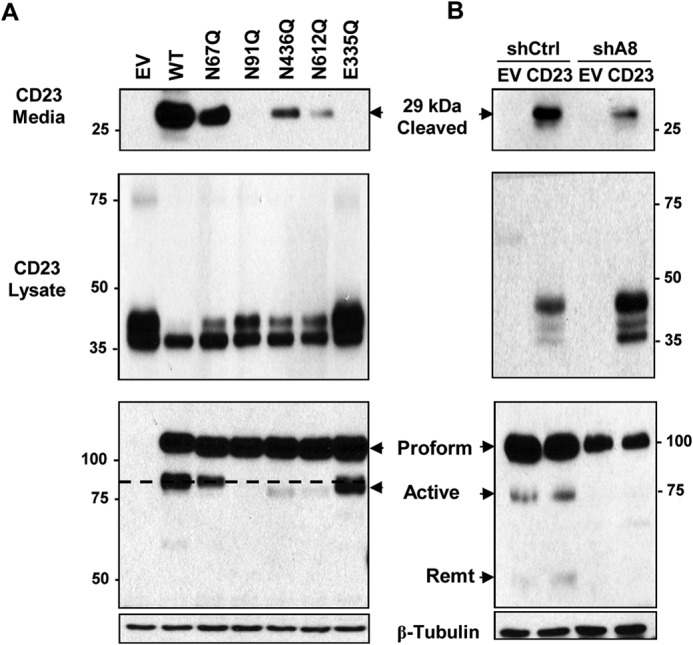
Glycosylation mutants with reduced processing display decreased ADAM8 metalloproteinase activity. A, C-terminal HA-tagged CD23 (CD23-HA) expression vector was co-transfected with EV DNA or with vectors expressing WT ADAM8, the indicated N-glycosylation mutants or metalloproteinase inactive E335Q mutant (EQ) in HEK-293 cells. 6 h later, the medium was replaced with serum-free medium. After 16 h, supernatants, and cells were collected. Medium samples were concentrated and assessed by Western blotting for cleaved CD23 (29 kDa) using an anti-HA antibody (top panel). Alternatively, WCEs were analyzed by Western blotting for CD23 expression using an HA antibody (middle panel) and for ADAM8 expression using a Myc antibody (bottom panels). The black dashed line indicates the migratory position of the active form of WT ADAM8. Note that the ADAM8-processed E335Q protein, while running near the position of active WT protein, has no detectable metalloproteinase activity, suggesting an alternative, non-functional enzymatic processing occurs in these cells. B, stable clones of MDA-MB-231 expressing shA8 RNA or control shRNA (shCtrl) were transfected with C-terminal HA-tagged CD23 (CD23-HA) vector DNA or EV DNA and processed and analyzed as in part A.
