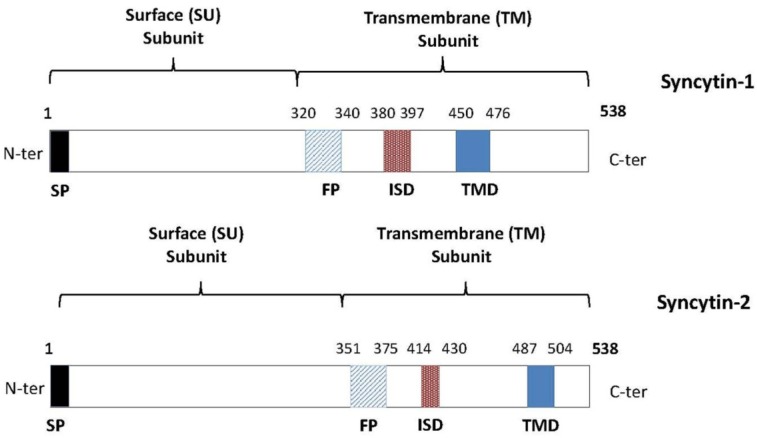
Figure 1.
Schematic presentation of the functional domains of Syncytin-1 and -2. Similarly to other retroviral glycoproteins, Syncytin-1 and Syncytin-2 are synthesized as inactive precursors, which are then cleaved into two functional subunits: the SU and the TM subunit. SU is responsible for receptor binding and TM mediates the fusion. Both proteins are 538 amino acids long and harbor a fusion peptide (FP), a functional immunosuppressive domain (ISD) and a transmembrane domain (TMD) in their TM subunit. As a membrane protein, the polyprotein also possesses a cleaved signal peptide (SP) at its amino end.